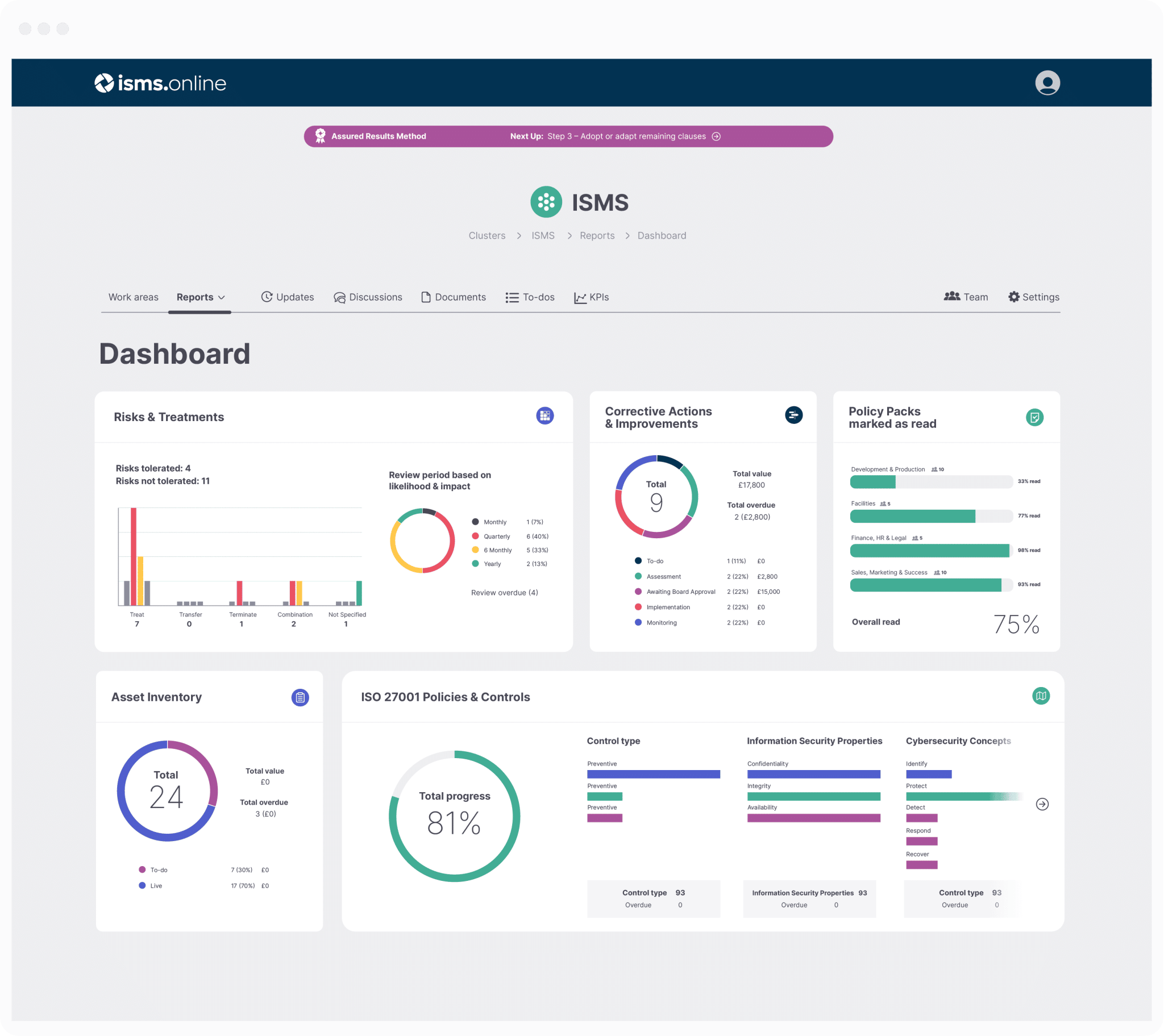
Implementing ISO standards in the medical industry and for medical device manufacturers is crucial for ensuring compliance with international best practices, streamlining processes, and enhancing patient safety and satisfaction.
These standards facilitate better risk management and help organisations meet legal requirements and industry standards. The adoption of ISO 9001 for quality management, ISO 45001 for occupational health and safety, and ISO 27001 for information security are particularly relevant for healthcare organisations.
By adhering to these standards, healthcare providers can reduce costs, improve employee training and development, and boost their reputation. Furthermore, compliance with ISO standards supports meeting Care Quality Commission (CQC) outcomes, leading to higher patient satisfaction rates and increased patient trust through secure data management.
Implementing these standards has resulted in millions of pounds saved by reducing workplace injuries and illnesses, as well as reduced operational risks and incidents.
ISMS.online offers a cloud-based compliance platform that simplifies ISO standard implementation, providing customisation solutions tailored to healthcare organisations. With expert guidance and support throughout the certification process, healthcare providers can efficiently achieve and maintain compliance with these essential standards, benefiting their organisation and patients.
Importance Of Compliance In The Medical Industry
Compliance in and among medical device manufacturers is crucial for various reasons. Patient safety is paramount, as adherence to regulatory requirements and ISO standards ensures the delivery of high-quality healthcare services.
Medical institutions and device manufacturers deal with sensitive data, such as patient records, clinical trial data, financial information, and proprietary design details, which require strong security measures to prevent unauthorised access and data breaches.
ISO standards implementation has been shown to improve overall performance in the healthcare sector.
Compliance fosters efficiency, trust, and credibility, essential for market access and maintaining a competitive edge. Continuous improvement and ethical business practices are integral to a sustainable organisational culture, reducing product recall risks and enhancing data security.
For instance, ISO 9001-certified organisations have reported a 57% reduction in customer complaints and a 55% improvement in on-time delivery.
Why ISO Standards Matter in the Medical Industry
ISO standards play a crucial role in the medical industry and medical device manufacturing by promoting continuous improvement, meeting regulatory requirements, facilitating global market access, encouraging collaboration, and ensuring patient safety. These standards provide a framework for organisations to develop and maintain quality management systems, risk management processes, and information security practices that align with international best practices.
Achieving ISO 27001 and other relevant ISO certifications through implementing an Integrated Management System (IMS) under the Annex SL structure can help these organisations adhere to strict regulations like the General Data Protection Regulation (GDPR) and other legal requirements.
The Benefits of ISO Certification for the Medical Industry
Adopting ISO certification in the medical industry and for medical devices, manufacturers offer numerous benefits. Firstly, it enhances operational efficiency by streamlining processes and reducing errors, leading to cost savings and improved patient outcomes. Continuous improvement is another advantage, as the ISO standards encourage organisations to regularly review and refine their systems, ensuring they remain up-to-date and effective.
Marketability is also improved, as ISO certification signals to potential clients and partners that an organisation is committed to quality and adheres to internationally recognised best practices. Furthermore, compliance with global regulatory requirements is facilitated, as ISO standards often align with or exceed the needs of various regulatory bodies, reducing the risk of non-compliance and associated penalties.
Key Benefits of an IMS Include:
Information Security: By implementing an IMS, healthcare organisations can protect their crucial information assets, ensuring confidentiality, integrity, and accessibility only to authorised individuals.
Attack Resilience: An IMS aids organisations in building resilience against various cyber threats, decreasing the likelihood of security breaches and minimising their impact.
Cost Reduction: Proactively managing information security risks with an IMS can help organisations mitigate potential financial losses from security breaches or non-compliance with regulations.
Security Threat Response: An IMS provides a centralised framework for identifying, assessing, and responding to security threats, allowing organisations to avoid emerging risks.
Compliance: Implementing an IMS can assist organisations in achieving compliance with various security standards and regulations, such as ISO standards and GDPR.
Integrated Management: By implementing an IMS, organisations can manage their quality, environmental, health and safety, and information security objectives in a coordinated and efficient manner.
Risk Management: An IMS allows organisations to identify, assess, and respond to various risks, including cyber threats, environmental hazards, and health and safety incidents.
Cost Savings: An IMS helps organisations proactively manage potential risks, thereby mitigating potential financial losses due to security breaches, non-compliance, or operational inefficiencies.
Regulatory Compliance: An IMS supports compliance with a range of international standards and regulations, such as ISO 9001, ISO 14001, ISO 45001, and ISO 27001, as well as GDPR.
Organisational Improvement: An IMS promotes continual improvement by providing a framework for setting, reviewing, and updating objectives based on performance data and changing circumstances.
An Introduction to Integrated Management Systems
Integrated Management Systems (IMS) for ISO standards offer a streamlined approach to managing multiple standards within a single system, simplifying management and promoting cross-functional collaboration.
Integrated Management Systems (IMS) are indispensable in preserving sensitive data, ensuring regulatory adherence, enabling secure collaboration, and augmenting operational efficiency in the healthcare industry. Healthcare providers, medical device manufacturers, and related entities manage confidential information such as patient records, research data, and proprietary device designs, which demand stringent security measures to prevent unauthorised access and data violations.
An efficient IMS enables secure and streamlined information exchange amongst these parties, minimising the risk of miscommunication and delays in care or production. An IMS can optimise processes and boost operational efficiency by automating routine tasks, centralising data management, and providing real-time insights into potential security risks.
Annex SL Explained
Annex SL is a high-level structure (HLS) that provides a consistent framework for ISO management system standards, enabling organisations to integrate multiple ISO standards more efficiently and effectively. This unified structure consists of identical core text, common terms, and definitions, facilitating the seamless integration of various ISO standards within an organisation’s management system.
Annex SL promotes a risk-based approach to decision-making, ensuring that organisations proactively identify, assess, and manage potential risks and opportunities. This approach not only improves the management system’s effectiveness but also fosters a culture of continuous improvement.
In summary, Annex SL plays a crucial role in simplifying the implementation and maintenance of ISO standards, ultimately contributing to an organisation’s long-term success and sustainability.
Enhancing Operational Efficiency and Compliance in the Medical Industry
Enhancing operational efficiency and compliance in the medical industry and among medical device manufacturers can significantly impact various aspects of their operations. By promoting a culture of quality and safety, organisations can ensure consistency in processes, leading to better decision-making and increased productivity.
- Streamlined communication allows quicker adaptation to changing regulations, building stakeholder trust and encouraging best practices.
- This contributes to a more resilient industry, potentially increasing market share and reducing errors and waste.
- Staying up-to-date with technological advancements and industry standards engages the workforce, demonstrating an ethical commitment to patient care.
- Supporting innovation drives growth and leads to a more efficient supply chain, ultimately benefiting the organisation and its patients.
Driving Continuous Improvement and Organisational Resilience
The medical industry and medical device manufacturers can drive continuous improvement and organisational resilience by adopting ISO standards, which provide a framework for implementing best practices and ensuring compliance with international regulations.
Proactive risk management enables organisations to identify potential hazards and implement preventive measures, reducing the likelihood of adverse events.
Fostering innovation encourages the development of new technologies and processes, enhancing the industry’s ability to adapt to changing demands and challenges. Strengthening supply chain management ensures the availability of high-quality materials and components, minimising disruptions and improving overall efficiency.
Benefits of ISMS.online
Utilising ISMS.online for the medical industry and medical device manufacturers offers numerous benefits in managing ISO compliance, enhancing data security, and streamlining certification processes. The platform’s comprehensive features facilitate adherence to international standards such as ISO 27001, ensuring robust information security management systems (ISMS) are in place. This safeguards sensitive patient data and helps organisations comply with stringent data protection regulations.
ISMS.online promotes a culture of quality and safety by providing a centralised, cloud-based platform for managing and monitoring compliance, enabling organisations to identify and mitigate risks effectively. The platform’s user-friendly interface and customisable templates simplify the certification process, reducing the time and resources required to achieve and maintain ISO compliance.
Commonly Used ISO Standards in the Medical Industry
The medical industry and medical device manufacturers rely on various ISO standards to ensure their operations’ quality, safety, and efficiency.
These standards provide a framework for assessing the bio-compatibility of devices, ensuring that they do not pose any undue risks to patients.
ISO 27001 – Information Security Management Systems Certification
The ISO 27001 Information Security Management System (ISMS) certification offers numerous benefits to the medical industry and medical device manufacturers:
- It protects sensitive patient data and intellectual property, crucial in maintaining trust and compliance with data protection regulations.
- It helps organisations identify and manage potential security risks, thereby reducing the likelihood of data breaches and the associated financial and reputational damages.
- ISO 27001 certification can improve business efficiency by streamlining processes and promoting a culture of continuous improvement.
ISO 27701 – The Information Privacy PIMS Standard Certification & GDPR Compliance
The ISO 27701 certification plays a crucial role in GDPR compliance for the medical industry, as it provides a comprehensive framework for managing personal information and ensuring data privacy. This standard complements ISO 27001 by extending its information security management system (ISMS) to include privacy-specific requirements, controls, and risk assessments.
By implementing ISO 27701, healthcare providers can demonstrate their commitment to protecting sensitive patient data and adhering to international privacy regulations, thereby strengthening trust and confidence among patients and stakeholders.
Moreover, ISO 27701 certification helps healthcare organisations identify and address potential privacy risks, ensuring that personal data is processed and stored securely. This reduces the likelihood of data breaches and minimises the potential financial and reputational damage associated with non-compliance.
ISO 22301 – The Business Continuity BCMS Standard
ISO 22301, the Business Continuity Management System (BCMS) standard, significantly benefits the medical industry and medical device manufacturers. It is crucial to ensure uninterrupted healthcare services by establishing a robust framework for identifying potential threats and developing strategies to mitigate their impact.
This proactive approach to risk management enhances organisational resilience, enabling healthcare providers and medical device manufacturers to maintain critical operations during disruptions, such as natural disasters, cyber-attacks, or supply chain issues.
ISO 9001 – Quality Management Systems Standard In The Medical Industry and Medical Device Manufacturers
The ISO 9001 Quality Management Systems Standard plays a crucial role in the medical industry and medical device manufacturing by providing a consistent framework for quality management. This framework ensures high-quality medical products and services, facilitates a customer-centric approach, and encourages continuous improvement and innovation.
By streamlining internal processes, ISO 9001 enhances collaboration and communication, supports the development of safe and effective medical devices, and demonstrates a commitment to quality and safety to stakeholders.
ISO 14001 – Environmental Management Systems
The ISO 14001 Environmental Management System (EMS) plays a crucial role in the medical industry and medical device manufacturing by reducing environmental impact and promoting sustainable practices.
This standard ensures compliance with environmental regulations and enhances corporate social responsibility (CSR) by systematically managing environmental aspects and impacts.
Medical organisations and device manufacturers adopting ISO 14001 can demonstrate their commitment to environmental stewardship, leading to improved stakeholder relations and a competitive advantage in the market.
ISO 45001 Occupational Health and Safety Management Systems
Implementing ISO 45001 Occupational Health and Safety Management Systems offers numerous benefits for the medical industry and medical device manufacturers. By enhancing workplace safety and promoting employee well-being, organisations can demonstrate their commitment to health and safety while supporting compliance with legal and regulatory requirements.
Integration with other ISO standards allows for a comprehensive risk management approach, helping identify and mitigate potential hazards. This proactive safety culture encourages effective incident reporting and response procedures, improving employee morale and productivity.
Furthermore, adopting ISO 45001 can enhance an organisation’s reputation, facilitating global market access and reducing operational downtime. This increased stakeholder trust supports sustainable business practices and contributes to overall success.
ISO 50001 – Energy Management System
Implementing ISO 50001 Energy Management System in the medical industry and medical device manufacturing offers numerous benefits.
Improved energy efficiency leads to reduced energy consumption, which lowers operational costs and contributes to a greener environment. Enhanced environmental performance is crucial for healthcare organisations, demonstrating their commitment to sustainable practices and helping them meet energy-related regulations.
This strengthened compliance with energy-related regulations reduces the risk of non-compliance penalties and fosters a positive public image.
Moreover, ISO 50001 certification increases market competitiveness, showcasing an organisation’s dedication to energy management and environmental responsibility.
ISO 13485 – Medical Devices Quality Management Systems Requirements for Regulatory Purposes
ISO 13485 is a globally recognised standard that establishes a comprehensive quality management system (QMS) for medical device manufacturers and suppliers.
This standard aims to ensure the safety and effectiveness of medical devices by providing a framework for the design, development, production, and maintenance of these products. One of the critical aspects of ISO 13485 is the emphasis on documentation and maintenance of procedures, which helps organisations maintain consistency and control throughout the product lifecycle.
Traceability and control of products throughout the supply chain are crucial components of ISO 13485, as they help to minimise risks and ensure that medical devices meet regulatory requirements. This standard also requires organisations to implement a risk management process in accordance with ISO 14971, which is a widely accepted standard for applying risk management to medical devices.
By adhering to ISO 13485 and ISO 14971, organisations can demonstrate their commitment to producing safe and effective medical devices while also meeting the expectations of regulatory authorities and customers worldwide.
Dedicated ISO standards In The Medical Industry
Various dedicated ISO standards govern the medical industry and medical device manufacturers; these standards guide quality and competence in medical laboratories, error reduction via risk management, sterilisation processes for healthcare products using ethylene oxide and radiation, and information provision for processing medical devices.
They also dictate the use of symbols on medical device labels, risk management applications in medical device production, and biological evaluation of these devices. Collectively, these standards ensure reliable laboratory results, safe sterilisation, clear device labelling, effective risk management, and safe use of medical devices, enhancing patient safety and treatment efficacy.
Examples include:
- ISO 15189 – Medical Laboratories – Requirements for Quality and Competence.
- ISO 22367 – Medical laboratories – Reduction of error through risk management and continual improvement.
- ISO 11135 – Sterilisation of health care products – Ethylene oxide – Requirements for the development, validation, and routine.
- ISO 11137 – Sterilisation of health care products – Radiation.
- ISO 17664 – Processing of health care products – Information to be provided by the medical device manufacturer for the processing of medical devices.
- ISO 15223-1 – Medical devices – Symbols to be used with medical device labels, labelling, and information to be supplied – Part 1: General requirements.
- ISO 14971 – Medical devices – Application of risk management to medical devices.
- ISO 10993 – Biological evaluation of medical devices.
- ISO 17664 – Processing of health care products – Information to be provided by the medical device manufacturer for the processing of medical devices.
Non-ISO Standards
In addition to ISO standards, the medical industry and medical device manufacturing are governed by various non-ISO standards and regulatory requirements that ensure patient safety, product efficacy, global market access, and continuous improvement. These include but are not limited to:
- IWA 1: Quality Management Systems Guidelines for Process Improvements in Health Service Organisations.
- American Society for Quality (ASQ) standards.
- Standards Council of Canada (SCC) standards.
- HIPAA (Health Insurance Portability and Accountability Act): US legislation that aims to protect the privacy and security of patient health information.
- USP 800 Hazardous Drugs: Handling in Healthcare Settings.
- USP 797 Pharmaceutical Compounding: Sterile Preparations.
- ANSI/AAMI ST79: Comprehensive Guide to Steam Sterilisation and Sterility Assurance in Health Care Facilities.
- ANSI/AAMI EC13: Cardiac Monitors, Heart Rate Meters, and Alarms.
- ASTM F2100: Standard Specification for Performance of Materials Used in Medical Face Masks.
- ASTM F1862: Standard Test Method for Resistance of Medical Face Masks to Penetration by Synthetic Blood (Horizontal Projection of Fixed Volume at a Known Velocity).
- CLSI M100: Performance Standards for Antimicrobial Susceptibility Testing.
Save Time and Money with ISMS.online
Utilising ISMS.online for your organisation’s compliance and certification needs offers significant time and cost savings. The platform streamlines the implementation of ISO standards by providing a centralised, cloud-based system that simplifies the management of multiple certifications. This reduces the administrative burden on your organisation and ensures a more efficient and organised approach to compliance.
Our expert consultants offer support throughout the certification process, enabling organisations to achieve certification in as little as 45 days. This accelerated timeline allows businesses to quickly demonstrate their commitment to internationally recognised best practices, enhancing their reputation and credibility in the market.
By streamlining the implementation process, centralising management, reducing administrative tasks, and accelerating certification timelines, the platform enables businesses to focus on delivering high-quality products and services to their customers.
Contact us today to book a demo.