ISO 9001, Clause 4.4, Quality Management System (QMS) and Its Processes
At the heart of the ISO 9001:2015 standard lies Clause 4.4, which focuses on the Quality Management System (QMS) and its processes. This clause is pivotal as it lays the foundation for establishing, implementing, maintaining, and continually improving a QMS. As we at ISMS.online understand, aligning your QMS with the organisation’s policy and strategic direction is not just a requirement; it’s a strategic enabler that drives consistency and improvement across all operational facets.
Understanding the Core of ISO 9001 Standard
The core of the ISO 9001 standard revolves around a process-oriented approach to quality management. This approach ensures that all processes are systematically managed and that they interact seamlessly to produce the desired outcomes. It’s about creating a structured framework that supports the delivery of products or services that consistently meet customer and regulatory requirements.
The Role of QMS in Organisational Alignment
A well-defined QMS is instrumental in aligning your business processes with the strategic goals of your organisation. It ensures that every member of your team understands their role in delivering quality and how it contributes to broader business objectives. This alignment is critical for driving efficiency and fostering a culture of continuous improvement.
Systematic Process Development and Continual Improvement
Our platform emphasises the importance of systematic process development, implementation, and continual improvement within your QMS. By adopting this approach, you’re not only complying with international standards but also positioning your organisation for long-term success through adaptive and responsive quality management practices.
The Concept of Interrelated Processes within the QMS
In a QMS, processes do not operate in isolation. They are interrelated and interdependent, forming a cohesive system that works towards a common goal. Understanding these interrelationships is key to managing and improving process efficiency and effectiveness, ultimately leading to enhanced customer satisfaction and business performance.Identifying and Defining Necessary QMS Processes
At ISMS.online, we understand that the heart of the ISO 9001:2015 standard lies in the Quality Management System (QMS) processes. To ensure that your organisation meets the highest standards of quality, it’s essential to identify and define the necessary QMS processes that are unique to your operational needs.
The Role of QMS Processes in Customer Satisfaction
Your commitment to customer satisfaction is reflected in how well your QMS processes are designed and implemented. By tailoring these processes to meet customer requirements effectively, you’re not just complying with ISO 9001:2015, but also enhancing the value you deliver to your customers.
Strategic Alignment with Organisational Goals
We advocate for a strategic approach where your QMS is aligned with your organisation’s policy and strategic direction. This ensures that every process is not only a step towards compliance but also towards achieving your long-term business objectives.
Continual Improvement in QMS Processes
Continual improvement is not just a requirement; it’s a mindset. By fostering an environment where every process is regularly evaluated and improved upon, you’re ensuring that your QMS remains dynamic and responsive to the ever-changing business landscape.
Remember, at ISMS.online, our platform is designed to support you in identifying, defining, and refining your QMS processes, ensuring they are always aligned with your strategic goals and customer satisfaction.
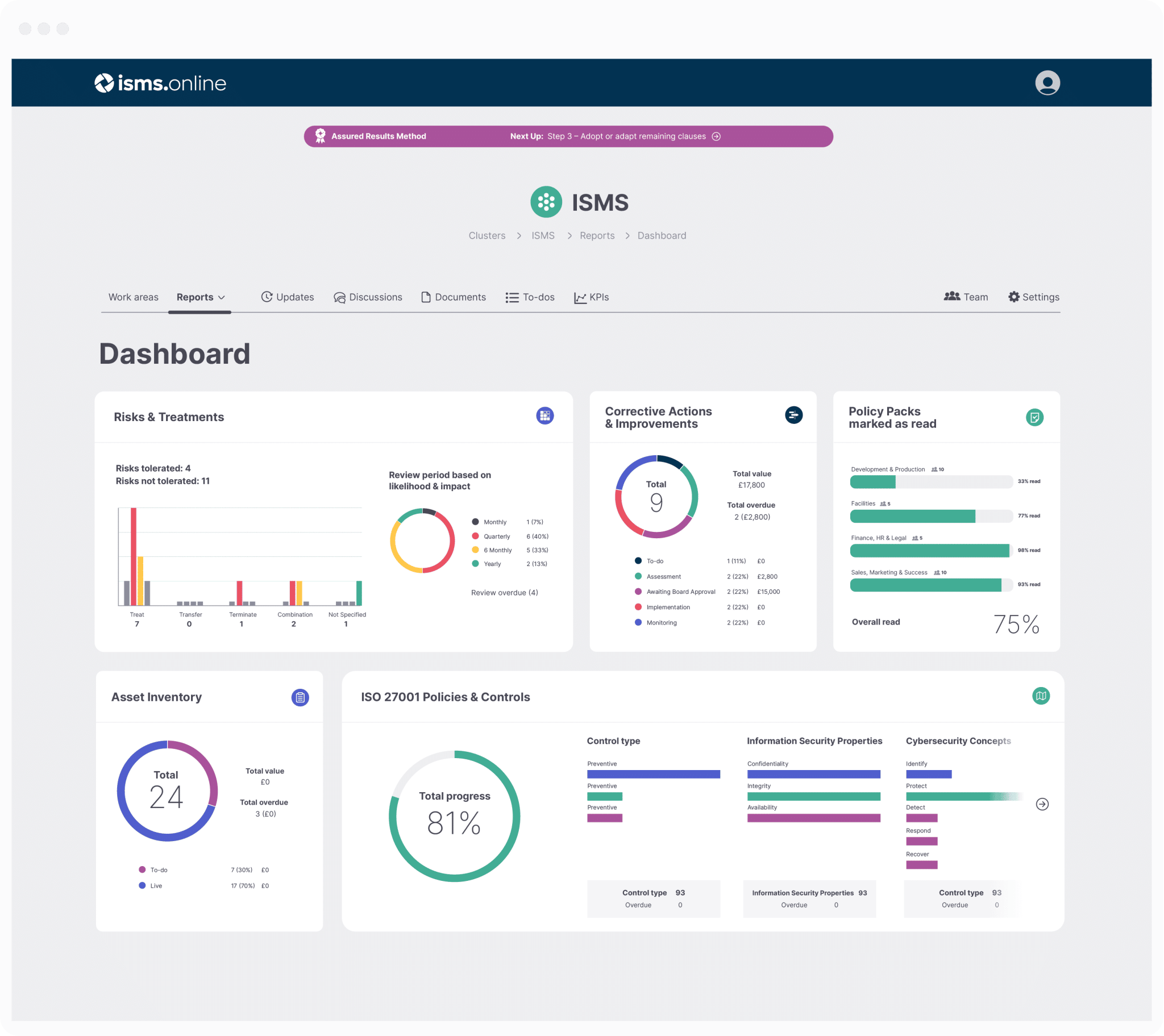
Get an 81% headstart
We've done the hard work for you, giving you an 81% Headstart from the moment you log on.
All you have to do is fill in the blanks.
Understanding the Key Elements of a Process
In the realm of ISO 9001:2015, Clause 4.4, every process within your Quality Management System (QMS) is a cog in a larger machine. At ISMS.online, we guide you through understanding the key elements that make your processes effective and compliant.
The Role of Inputs and Outputs in a Process
Each process you implement is driven by inputs, which can be physical or informational, and leads to outputs, such as a product or service. recognising the nature and quality of these inputs and outputs is crucial for the success of your QMS.
Sequence and Interaction of Processes
Processes do not exist in isolation. The sequence in which they operate and their interaction with one another form a complex network that must be carefully mapped out to ensure efficiency and effectiveness.
Control in Managing Processes
Control mechanisms are vital in overseeing these processes. They ensure that each step is performed correctly and consistently, leading to the intended results. At ISMS.online, our platform provides you with the tools to establish such controls effectively.
Defining and Measuring Intended Results
Finally, understanding how to define and measure the intended results of your processes is essential. This involves setting clear objectives and using performance indicators to track progress and implement improvements where necessary.
Detailed Requirements of Sub-clauses 4.4.1 and 4.4.2
When you delve into the specifics of ISO 9001:2015, sub-clauses 4.4.1 and 4.4.2 serve as the blueprint for maintaining and enhancing your Quality Management System (QMS). At ISMS.online, we’re committed to helping you comprehend and implement these detailed process requirements.
The Role of Environment, Control, and Review
These sub-clauses emphasise the significance of the environment in which the QMS operates, the control measures that govern it, and the review mechanisms that ensure its continual suitability and effectiveness. They are pivotal in:
- Establishing a conducive environment for the processes to function optimally.
- Implementing control measures to maintain process integrity.
- Regularly reviewing processes to align with dynamic business and customer needs.
Ensuring QMS Effectiveness
To ensure the effectiveness of your QMS, sub-clauses 4.4.1 and 4.4.2 guide you to:
- Maintain documented information to support operation and monitoring.
- Apply a systematic approach to manage and improve processes.
- Engage in continual review and control to adapt to internal and external changes.
Contribution to the Overall QMS Framework
These sub-clauses are not standalone; they integrate into the overall QMS framework, reinforcing the foundation for a robust and resilient management system. With our platform, you’re equipped to seamlessly incorporate these requirements into your QMS, ensuring a comprehensive approach to quality management.
Manage all your compliance in one place
ISMS.online supports over 100 standards
and regulations, giving you a single
platform for all your compliance needs.
Addressing Risks and Opportunities in QMS
In the pursuit of excellence within your Quality Management System (QMS), ISO 9001:2015, Clause 4.4, places a strong emphasis on the proactive management of risks and opportunities. At ISMS.online, we provide the framework to ensure that you’re not only compliant but also resilient in the face of potential challenges.
Identifying and Managing Internal and External Issues
To safeguard the integrity of your QMS, it’s imperative to:
- Identify both internal and external issues that could impact your processes.
- analyse these issues to understand their potential effects.
- Develop strategies to mitigate risks and capitalise on opportunities.
Engaging Interested Parties in Risk Management
Your QMS doesn’t operate in a vacuum. Engaging interested parties, such as customers, employees, and suppliers, is key to:
- Gaining insights into potential risks and opportunities.
- Ensuring that your risk management strategies are comprehensive and effective.
Impact on QMS Effectiveness
By systematically addressing risks and opportunities, you enhance the effectiveness of your QMS by:
- Preventing or reducing undesired impacts and potential failures.
- Improving on existing processes and outcomes.
- Ensuring continual improvement and customer satisfaction.
With our platform, you’re equipped to navigate the complexities of risk and opportunity management, ensuring that your QMS remains robust and responsive to the needs of all stakeholders.
The Role of Documented Information in QMS
In the context of ISO 9001:2015, Clause 4.4, documented information is the backbone of a robust Quality Management System (QMS). At ISMS.online, we emphasise the critical role that such documentation plays in supporting the operation and effectiveness of your QMS processes.
Types of Documented Information in QMS
Documented information within your QMS can take various forms, all of which serve to solidify your processes:
- Operational Procedures: These are the step-by-step instructions that ensure consistency in your operations.
- Work Instructions: More detailed than procedures, they guide your team through complex tasks.
- Flow Charts: Visual representations of your processes that enhance understanding and training.
- Corrective Actions: Documentation that addresses and rectifies any non-conformities.
Ensuring Confidence in Process Execution
Documented information provides the assurance that your processes are not only planned but also executed as intended. This is crucial for:
- Maintaining Quality: Ensuring that every output meets the required standards.
- Facilitating Audits: Providing clear evidence for internal and external audits.
Importance of Document Maintenance
Keeping your documented information up-to-date is not optional; it’s a necessity for:
- Reflecting Changes: Ensuring your documentation evolves with your processes.
- Continuous Improvement: Leveraging documentation for ongoing enhancements to your QMS.
By maintaining and regularly reviewing your documented information, you ensure that your QMS remains a true reflection of your commitment to quality.
Compliance doesn't have to be complicated.
We've done the hard work for you, giving you an 81% Headstart from the moment you log on.
All you have to do is fill in the blanks.
Managing Outsourced Processes
In today’s interconnected business environment, outsourcing is a reality for many organisations. However, when it comes to your Quality Management System (QMS), the control and integration of outsourced processes are critical to meet ISO 9001:2015 requirements. At ISMS.online, we provide the expertise to ensure that these external processes complement your internal efforts seamlessly.
Defining and Documenting Outsourced Processes
To maintain the integrity of your QMS:
- Define the scope and objectives of each outsourced process clearly.
- Document these processes meticulously, ensuring they align with your QMS standards.
Implementing Purchasing Process Controls
Effective management of outsourced processes involves:
- Establishing purchasing controls to ensure suppliers meet your quality requirements.
- Regularly evaluating supplier performance to maintain QMS standards.
Impact on QMS Effectiveness
Outsourced processes can significantly influence the effectiveness of your QMS by:
- Extending your quality standards beyond the immediate boundaries of your organisation.
- Potentially introducing risks that need to be managed proactively.
Through our platform, you’re empowered to integrate outsourced processes into your QMS, ensuring they contribute positively to your overall quality objectives.
Further Reading
The Connections Between ISO 9001 Clauses
Navigating the complexities of ISO 9001:2015 can be daunting, but at ISMS.online, we recognise that understanding the interconnections between various clauses is key to simplifying this journey. Each clause is a thread in the fabric of your Quality Management System (QMS), and when woven together, they create a cohesive and robust system.
Simplifying Compliance Through Clause Interconnections
By understanding how different clauses of ISO 9001 interrelate, you can:
- Streamline your compliance efforts, avoiding duplication of work.
- Ensure that changes in one area are reflected across your QMS, maintaining consistency.
Ensuring QMS Effectiveness
The interconnections play a pivotal role in:
- Aligning your QMS processes with the strategic direction of your organisation.
- Facilitating a holistic approach to managing quality, where each process supports the others.
Impact on Continual Improvement Efforts
These interconnections are not static; they are dynamic pathways that support:
- The identification of areas for improvement.
- The implementation of changes that lead to enhanced performance and customer satisfaction.
With our platform, you’re equipped to understand and leverage these interconnections, ensuring that your QMS is not only compliant but also continuously improving.
The Role of ISMS.online in Achieving ISO 9001 Compliance
Navigating the complexities of ISO 9001:2015 compliance can be a challenging endeavour. At ISMS.online, we pride ourselves on providing a comprehensive platform that simplifies this process for you. Our goal is to ensure that your Quality Management System (QMS) not only meets the standards but does so with efficiency and ease.
Key Features Supporting ISO 9001 Compliance
Our platform is equipped with a suite of features designed to streamline your QMS processes:
- Pre-configured QMS: Jumpstart your compliance journey with our ready-to-use QMS templates.
- Assured Results Method (ARM): Follow our structured approach to achieve and maintain compliance.
- AAA Framework: Access, Accountability, and Assurance are at the core of our platform, providing a clear pathway to compliance.
Benefits of Using ISMS.online for QMS Management
By choosing ISMS.online, you’re opting for:
- Efficient Document Management: organise and control your documented information with ease.
- Dynamic Risk Management Tools: Identify, assess, and manage risks effectively.
- Robust Policy and Control Management: Ensure that your policies are up-to-date and controls are enforced.
Ensuring Continual Improvement in QMS
We understand that compliance is not a one-time event but a continuous journey. Our platform is designed to facilitate ongoing improvement, helping you to:
- Stay ahead of changes with simplified audits and reviews.
- Engage with interested party management for a holistic approach to quality.
- Achieve transparent reporting for clear insights into your QMS performance.
With ISMS.online, you’re not just complying with ISO 9001; you’re setting a foundation for excellence in quality management.
Understanding the Context of the Organisation and QMS Scope
When implementing a Quality Management System (QMS), comprehending the context of your organisation is paramount. At ISMS.online, we emphasise that this understanding is not merely about compliance; it’s about aligning your QMS with the unique aspects of your business environment.
Defining the QMS Scope Based on Organisational Context
To define the scope of your QMS effectively, consider:
- The external and internal factors that influence your objectives and the direction of your processes.
- The specific requirements of your products and services, and how they impact your QMS design.
The Role of Stakeholders in QMS Scope Definition
Engaging stakeholders is crucial in shaping your QMS:
- Identify who your interested parties are, from customers to suppliers, and understand their needs and expectations.
- Incorporate their feedback to ensure your QMS is responsive and relevant.
Impact on ISO 9001 Compliance
The context of your organisation and the defined scope of your QMS have a direct impact on your ISO 9001 compliance efforts:
- They determine the boundaries and applicability of your management system.
- They ensure that your QMS is tailored to your operational reality, enhancing its effectiveness.
By considering these factors, you’re not just meeting ISO 9001 standards; you’re creating a QMS that truly reflects the essence of your organisation.
The Importance of Quality Indicators and Management Review in QMS
In the pursuit of excellence within your Quality Management System (QMS), it’s essential to establish and monitor quality indicators. At ISMS.online, we provide the tools and guidance to ensure these indicators effectively reflect the health of your QMS processes.
Utilising Quality Indicators for QMS Monitoring
Quality indicators serve as the compass that guides your QMS. They help you to:
- Monitor the performance of your processes in real-time.
- Control the quality by identifying variances and implementing corrective actions promptly.
Conducting Effective Management Reviews
Management reviews are not just a formality; they are a critical evaluation tool. During these reviews, you should:
- Assess the effectiveness of your QMS against set Key Performance Indicators (KPIs).
- Ensure that your QMS is continually improving and meeting the objectives.
Integrating KPIs into Management Reviews
To leverage KPIs effectively:
- Choose indicators that are aligned with your quality objectives.
- Use them to drive decisions that enhance customer satisfaction and process efficiency.
Promoting Quality Goals Awareness
Management reviews also play a vital role in:
- Reinforcing the importance of quality goals across your organisation.
- Ensuring that every team member is aligned with these goals and understands their role in achieving them.
By focusing on these aspects, you ensure that your QMS is not only compliant but also a catalyst for continuous improvement and organisational excellence.
Contact ISMS.online for ISO 9001 Clause 4.4 Compliance
Navigating the intricacies of ISO 9001:2015, particularly Clause 4.4, can be complex. At ISMS.online, we are dedicated to providing clarity and support throughout your QMS journey. If you’re seeking further information or guidance, our team is ready to assist.
Benefits of Contacting ISMS.online for ISO 9001 Support
When you reach out to us, you’ll gain access to:
- Expert Guidance: Our team has in-depth knowledge of ISO 9001 requirements and best practices.
- Tailored Solutions: We understand that every organisation is unique, and we’re here to provide solutions that fit your specific needs.
Our Services for Achieving ISO 9001 Compliance
We offer a range of services to facilitate your compliance:
- Pre-configured QMS Templates: Simplify the process of setting up your QMS with our ready-to-use templates.
- Risk Management Tools: Identify and manage potential risks effectively with our comprehensive tools.
Our Role in Your ISO 9001 Compliance Journey
Our platform is designed to support you every step of the way by:- Streamlining Documentation: organise and maintain your QMS documentation with ease.
- Facilitating Continuous Improvement: Use our tools to continually refine and enhance your QMS processes.
For more information on how we can assist you with ISO 9001:2015, Clause 4.4, and your QMS, please contact us. We're here to help you achieve and maintain excellence in quality management.