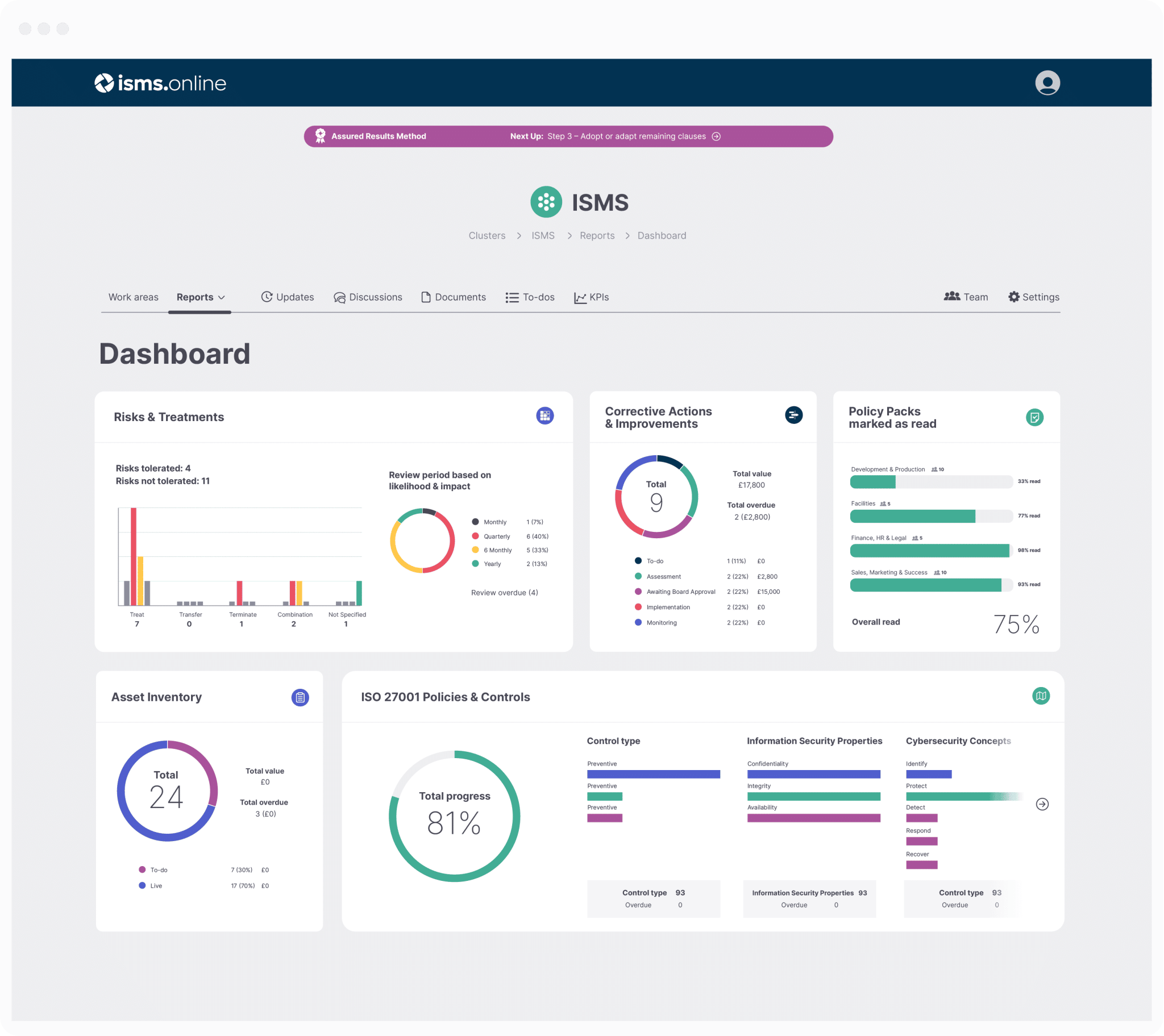
ISO 9001, Clause 9, Explained
Annex SL is a pivotal structure that underpins the ISO management system standards, including ISO 9001:2015. It provides a universal high-level framework that fosters consistency and harmonisation across various standards, which is particularly beneficial for organisations implementing multiple management systems.
Key Elements of Annex SL Relevant to Performance Evaluation
Annex SL introduces a set of core definitions and standardised text that ensures uniformity in the clauses of different ISO standards. For performance evaluation, this means that the processes for monitoring, measurement, analysis, and evaluation are consistent, allowing for a more streamlined approach to managing and improving organisational performance.
Enhancing Integration of ISO Management Standards
By adopting Annex SL, ISO 9001:2015 aligns with other management system standards, facilitating an integrated approach. This integration is crucial for organisations that aim to comply with multiple standards, as it simplifies the implementation and maintenance of their management systems.
Benefits of a standardised Framework
A standardised framework like Annex SL provides several benefits:- Simplified Certification: It eases the process of obtaining and maintaining ISO certification by reducing complexity.
- Efficient Resource utilisation: organisations can allocate resources more efficiently by avoiding duplication of efforts across different standards.
- Improved organisational Agility: It enables quicker adaptation to changes in compliance requirements.
At ISMS.online, we understand the importance of a standardised approach. Our platform is designed to help you seamlessly integrate your QMS with other management systems, ensuring that your performance evaluation processes are robust and aligned with the strategic direction of your organisation.
Monitoring and Measurement Strategies
When it comes to ISO 9001:2015, Clause 9 emphasises the importance of monitoring and measurement as a foundation for effective performance evaluation. As you navigate this clause, it’s essential to understand the strategic approach to these activities.
Determining What to Monitor and Measure
Initially, you must identify the processes that are critical to your quality objectives. This involves considering customer requirements, regulatory compliance, and your organisation’s strategic goals. At ISMS.online, we provide a framework that helps you align these considerations with your monitoring activities to ensure they are both relevant and effective.
Ensuring Valid and Reliable Results
To guarantee the validity and reliability of your performance data, it’s crucial to employ robust methods and tools. We recommend using standardised procedures and calibrated instruments to collect data. Our platform can assist in maintaining the integrity of your data through secure storage and controlled access, ensuring that the information you rely on for decision-making is both accurate and trustworthy.
Scheduling Monitoring and Measurement Activities
Effective scheduling is key to consistent performance evaluation. It’s important to establish a regular cadence for these activities that aligns with your operational cycles and allows for timely analysis. Our platform facilitates the scheduling of monitoring and measurement tasks, ensuring that they are conducted at appropriate intervals and that the results are available when you need them.
Streamlining Strategies with ISMS.online
At ISMS.online, we understand the complexities of performance evaluation. Our platform is designed to streamline your monitoring and measurement strategies, integrating seamlessly with your existing processes and providing a centralised hub for all your performance data. With our tools, you can schedule, collect, and analyse data with ease, freeing you to focus on the continual improvement of your QMS.
Get an 81% headstart
We've done the hard work for you, giving you an 81% Headstart from the moment you log on.
All you have to do is fill in the blanks.
Data Collection and Analysis
In the realm of ISO 9001:2015, Clause 9, data collection and analysis form the backbone of an effective Quality Management System (QMS). Understanding what data to collect and how to analyse it is crucial for ensuring your QMS is performing as expected.
Essential Data for QMS Performance Evaluation
To evaluate your QMS performance effectively, you need to collect data that is:
- Relevant to Quality Objectives: This ensures alignment with your strategic goals.
- Reflective of Process Performance: To monitor the efficiency and effectiveness of your processes.
- Based on Customer Feedback: Customer satisfaction is a key indicator of quality.
- Compliant with Audit Results: Audits provide insights into the conformance of your QMS.
Aligning Data Collection with Quality Objectives
To align data collection with your quality objectives, consider:
- Defining Clear Metrics: Establish what success looks like for each objective.
- Using standardised Methods: Ensure consistency and reliability in the data collected.
Best practices for analysing and Evaluating QMS Data
When analysing and evaluating your QMS data, it’s important to:
- Regularly Review Data: Frequent analysis can help spot trends and areas for improvement.
- Use Statistical Techniques: These can provide deeper insights into your QMS performance.
Enhancing Data Collection and Analysis with ISMS.online
At ISMS.online, we provide tools that enhance your data collection and analysis processes by:
- centralising Data Storage: Keep all your data in one secure, accessible location.
- Automating Data Collection: Reduce manual errors and save time.
- Providing Analytical Tools: Gain actionable insights with built-in analytics features.
By leveraging our platform, you can ensure that your data collection and analysis are not only aligned with ISO 9001:2015 standard but also optimised for continuous improvement.
Customer Satisfaction Assessment
Understanding and measuring customer satisfaction is a cornerstone of the ISO 9001:2015 standard. It’s not just about meeting specifications; it’s about exceeding customer expectations and continually improving their experience with your products or services.
Effective Methods for Obtaining Customer Satisfaction Data
To gauge customer satisfaction effectively, you should employ a variety of methods:
- Surveys and Questionnaires: These can provide quantitative data on customer satisfaction levels.
- Feedback Forms: Offering a simple way for customers to provide their thoughts can yield qualitative insights.
- Direct Communication: Engaging with customers through interviews or focus groups can uncover deeper feedback.
Incorporating Customer Feedback into the QMS
Once collected, customer feedback should be analysed and used to:
- Identify Areas for Improvement: Look for common themes or recurring issues in the feedback.
- Set Actionable Goals: Use the insights to inform your quality objectives and drive improvements.
ISMS.online’s Support in Monitoring Customer Perceptions
At ISMS.online, we provide tools that help you:
- Collect Feedback Efficiently: Our platform can streamline the distribution and collection of surveys and feedback forms.
- analyse Data Effectively: With built-in analytics, you can quickly identify trends and areas for action.
- Document Changes: Keep a record of how customer feedback has influenced your QMS, demonstrating a commitment to continual improvement.
By prioritising customer satisfaction and leveraging our platform, you can ensure that your QMS remains customer-focused and aligned with ISO 9001:2015 standard.
Manage all your compliance in one place
ISMS.online supports over 100 standards
and regulations, giving you a single
platform for all your compliance needs.
Internal Audit
Conducting an internal audit is a fundamental requirement of ISO 9001:2015, serving as a mirror to reflect the health of your Quality Management System (QMS). It’s a moment of truth where you assess if your processes align with the established standards and your own quality objectives.
Objectives of Conducting an Internal Audit
The primary objectives of an internal audit within the QMS framework are to:
- Verify Compliance: Ensure that your practices conform to both ISO 9001 standard and internal requirements.
- Assess Effectiveness: Evaluate if the implemented processes are effective in achieving the desired quality outcomes.
- Identify Opportunities for Improvement: Uncover areas where your QMS can be enhanced for better performance.
Gathering Objective Evidence
To gather objective evidence, you should:
- Conduct Interviews: Engage with personnel to understand their awareness and implementation of QMS processes.
- Review Documents and Records: Examine the documented information to verify that it reflects actual practices.
- Observe Processes: Witness operations to see if they are carried out as prescribed by your QMS.
Characteristics of an Effective Internal Audit programme
An effective internal audit programme is characterised by:
- Regular Scheduling: Audits should be conducted at planned intervals to ensure continuous compliance.
- Trained Auditors: Auditors must be competent and understand the audit criteria, scope, and techniques.
- Impartiality: Auditors should be objective and free from bias to ensure the integrity of the audit findings.
ISMS.online’s Assistance in Conducting Audits
At ISMS.online, we provide a platform that supports you in:
- Planning Audits: Schedule and track audit activities with ease.
- Documenting Findings: Record observations systematically for traceability.
- Implementing Corrective Actions: Manage follow-up activities to address any identified non-conformities.
By utilising our system, you can ensure that your internal audits are thorough, unbiased, and contribute to the continual improvement of your QMS.
The Role of Management
Management review is a structured process that is essential for the continuous improvement of a Quality Management System (QMS). It is a strategic exercise that ensures your QMS remains effective and aligned with the overall business objectives.
Key Components of a Management Review
Under ISO 9001, a management review must address several key components:
- Performance Evaluation: Reviewing the performance of the QMS against the set objectives and metrics.
- Resource Adequacy: Ensuring that the necessary resources are available to maintain and improve the QMS.
- Opportunities for Improvement: Identifying potential changes to the QMS that could enhance performance.
Strategic Alignment and Continual Improvement
The management review process is integral to:
- Aligning the QMS with Business Strategy: It ensures that the QMS evolves in tandem with the strategic direction of the organisation.
- Driving Continual Improvement: By regularly assessing the QMS, management can make informed decisions that foster ongoing enhancement.
Documented Evidence for Management Reviews
To demonstrate the effectiveness of management reviews, you must maintain:
- Records of Meetings: Documenting the discussions and decisions made during the reviews.
- Action Plans: Keeping track of the actions taken as a result of the review findings.
ISMS.online’s Facilitation of the Management Review Process
At ISMS.online, we provide a platform that simplifies the management review process by:
- centralising Documentation: Allowing you to maintain a clear record of each review.
- Tracking Actions: Enabling you to monitor the progress of improvements and ensure accountability.
By leveraging our platform, you can ensure that your management reviews are thorough, effective, and contribute to the strategic success of your QMS.
Compliance doesn't have to be complicated.
We've done the hard work for you, giving you an 81% Headstart from the moment you log on.
All you have to do is fill in the blanks.
Addressing Non-Conformity and Corrective Actions
Identifying and addressing non-conformities within your Quality Management System (QMS) is a critical step in maintaining the integrity of your ISO 9001:2015 certification. It’s about taking proactive measures to prevent issues from recurring and driving continuous improvement.
Identifying and Documenting Non-Conformities
Non-conformities are deviations from the set QMS procedures or standards. To effectively manage these:
- Conduct Thorough Inspections: Regularly review processes to detect any deviations.
- Document Findings: Record the non-conformity details, including the nature of the deviation and its potential impact on quality.
Investigating and Preventing Recurrence
Once a non-conformity is identified, the next steps are crucial:
- Root Cause Analysis: Investigate to understand why the non-conformity occurred.
- Implement Corrective Actions: Develop and apply measures to prevent recurrence.
Driving Continual Improvement
Corrective actions are not just about fixing problems; they’re about learning from them:
- Review Effectiveness: Assess whether the corrective actions have resolved the issues.
- Update QMS Documentation: Reflect any changes made to prevent future non-conformities.
ISMS.online’s Role in Managing Non-Conformity
At ISMS.online, we provide tools to help you manage non-conformities effectively:
- Structured Workflows: Guide you through the process of documenting and addressing non-conformities.
- Action Tracking: Monitor the implementation and effectiveness of corrective actions.
- Audit Trails: Maintain a clear record of all steps taken, supporting transparency and accountability.
By utilising our platform, you can ensure that non-conformities are handled efficiently, leading to a stronger and more resilient QMS.
Further Reading
Risk Management Integration in Performance Evaluation
Risk management is an integral component of the ISO 9001:2015 standard, particularly within the context of performance evaluation. It requires a proactive approach to identifying potential risks and opportunities that could impact the Quality Management System (QMS).
Identifying and Addressing Risks and Opportunities
To effectively integrate risk management into your performance evaluation, you should:
- Conduct Risk Assessments: Regularly evaluate your processes to identify potential risks to your QMS.
- Analyse Risk Impact: Determine the potential consequences of identified risks on your operations and quality objectives.
Assessing and Mitigating the Impact of Risks
Once risks are identified, it’s crucial to:
- Prioritise Risks: Focus on risks that could have the most significant impact on your QMS.
- Develop Mitigation Strategies: Implement controls to minimise the likelihood or impact of these risks.
Continuous Improvement Through Risk Management with ISMS.online
At ISMS.online, we support your continuous improvement efforts by providing:
- Risk Management Tools: Our platform includes features that help you identify, assess, and manage risks effectively.
- Actionable Insights: We enable you to translate risk assessments into concrete actions for improvement.
- Comprehensive Documentation: Maintain clear records of your risk management activities, supporting accountability and informed decision-making.
By leveraging our platform, you can ensure that risk management is a seamless part of your performance evaluation, contributing to the resilience and effectiveness of your QMS.
Documenting Information
Maintaining meticulous records is a cornerstone of ISO 9001 compliance. Documented information provides the evidence needed to demonstrate the effectiveness of your Quality Management System (QMS) and is essential during audits.
Types of Documented Information for ISO 9001 Compliance
For ISO 9001 compliance, you’re required to maintain:
- Quality Policy and Objectives: Clearly stating your commitment to quality.
- Scope of the QMS: Outlining the boundaries and applicability of your QMS.
- Evidence of Monitoring and Measurement: Records that demonstrate your QMS’s performance.
- Audit Reports and Management Reviews: Documenting the continual assessment and improvement of your QMS.
Managing Records of Audits, Reviews, and Corrective Actions
Effective record management involves:
- Organised Storage: Keeping records in a systematic manner for easy retrieval.
- Accessibility: Ensuring that relevant personnel can access records when needed.
- Retention and Disposal: Adhering to defined periods for keeping records and secure disposal methods.
The Importance of Documented Information During Auditing
Documented information is your proof of compliance and commitment to quality. It:
- Demonstrates Conformance: Shows auditors that your processes meet ISO 9001 standard.
- Provides Traceability: Allows for the tracking of changes and decision-making processes.
ISMS.online’s Role in Efficient Document Management
At ISMS.online, we streamline your record-keeping by:
- Centralising Documentation: Offering a single repository for all your QMS records.
- Automating Workflows: Simplifying the capture and maintenance of records.
- Enhancing Security: Protecting sensitive information with robust security measures.
By utilising our platform, you ensure that your documented information is managed efficiently, supporting the integrity and success of your QMS.
Efficient Document Management
Efficient document management is a linchpin in the successful implementation and maintenance of a Quality Management System (QMS). In today’s digital age, the integration of applications plays a significant role in streamlining this process.
Benefits of App Integration for Document Management
Integrating applications into your QMS can:
- Improve Accessibility: Ensure that documents are readily available to authorised personnel, regardless of their location.
- Enhance Collaboration: Facilitate real-time collaboration across different departments and teams.
- Increase Efficiency: Automate routine tasks, reducing the time spent on manual document handling.
Utilising Tools like Microsoft SharePoint and Google Drive
Platforms such as Microsoft SharePoint and Google Drive can be utilised to:
- Store Documents Centrally: Keep all QMS documentation in one secure, organised place.
- Control Document Versions: Manage updates and revisions effectively, ensuring that everyone uses the most current documents.
- Facilitate Workflow Management: Streamline processes with built-in workflow capabilities.
ISMS.online’s Seamless Integration with Over 5000 Apps
At ISMS.online, we pride ourselves on providing a platform that:
- Supports Extensive App Integration: Connect with over 5000 apps to tailor your QMS to your unique needs.
- Simplifies Document Control: Our platform’s integration capabilities make managing your QMS documentation effortless.
- Enhances QMS Effectiveness: By integrating with the tools you already use, we help make your QMS more effective and user-friendly.
Through our platform, we ensure that you have all the necessary tools at your disposal to maintain a robust and compliant QMS.