ISO 9001, Clause 8.4 Explained
As you delve into the ISO 9001:2015 standard, it’s essential to recognise the evolution of Clause 8.4 from its 2008 predecessor. Our platform, ISMS.online, is committed to helping you navigate these changes effectively.
Significant Changes from ISO 9001:2008 to ISO 9001:2015
The definition of ‘external provider’ has been broadened to include all forms of external outsourcing, not just those traditionally considered suppliers. This expansion necessitates a more inclusive approach to supplier management, ensuring that every external source meets the rigorous standards of your Quality Management System (QMS).
Impact on Evaluation and Selection of Suppliers
With the updated clause, the evaluation and selection process for suppliers must now be more thorough, incorporating a wider range of criteria to ensure alignment with your organisation’s quality objectives. This means that you’re not just assessing the quality of products and services, but also the processes and interactions these providers have with your QMS.
Evolution of Record-Keeping for Supplier Activities
Record-keeping has become more stringent under the new clause. You must now document not only the criteria for supplier activities but also the results of these activities, ensuring a traceable audit trail that demonstrates compliance and facilitates continuous improvement.
Enhancing Efficiency and Risk Mitigation
These changes are designed to enhance the efficiency of your supply chain and mitigate risks associated with external provisions. By maintaining comprehensive records and aligning verification activities with your quality objectives, you can prevent reputational damage and financial losses, safeguarding the integrity of your QMS.Strategies for Supplier Management
Effective supplier management hinges on meticulous record-keeping. As a compliance officer, you’re tasked with ensuring that your organisation’s supplier records are both comprehensive and compliant with ISO 9001:2015 Clause 8.4.
Criteria for Documenting Supplier Activities
When documenting supplier activities, it’s crucial to include:
- Supplier selection criteria: Clearly define what makes a supplier suitable for your organisation.
- Evaluation results: Record outcomes of supplier assessments, focusing on their ability to meet your quality requirements.
- Performance data: Keep track of ongoing supplier performance metrics.
Ensuring Accurate and Comprehensive Records
To ensure records are both accurate and comprehensive, consider:
- Regular updates: Keep supplier information current to reflect any changes or new evaluations.
- Cross-functional input: Involve various departments in the evaluation process for a holistic view of supplier performance.
The Importance of Record-Keeping
Record-keeping is not just a procedural necessity; it’s a cornerstone of your QMS. It provides:
- Evidence of due diligence: Demonstrates your commitment to quality and compliance.
- Basis for improvement: Identifies areas for supplier development and QMS enhancement.
ISMS.online’s Role in Document Management
At ISMS.online, we understand the importance of efficient document management. Our platform offers:
- Integrated tools: utilise our document management features to streamline the creation, storage, and retrieval of supplier records.
- Compliance support: Our system is designed to help you maintain records that are in line with ISO 9001:2015 requirements, ensuring your QMS remains robust and transparent.
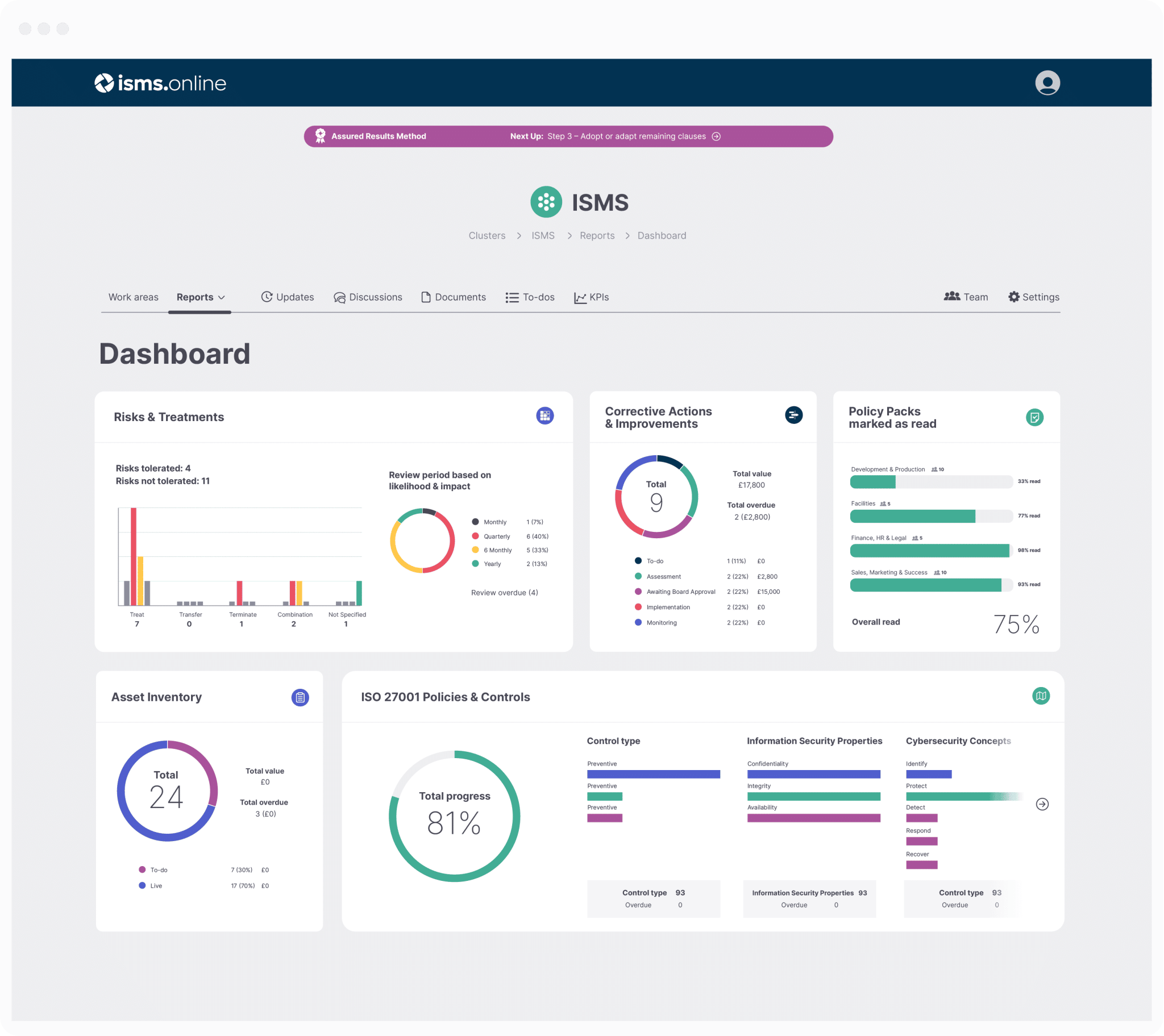
Get an 81% headstart
We've done the hard work for you, giving you an 81% Headstart from the moment you log on.
All you have to do is fill in the blanks.
Aligning Verification Activities
To maintain the integrity of your Quality Management System (QMS), it’s imperative that verification activities are closely aligned with your organisation’s quality objectives. This alignment ensures that externally provided processes, products, and services contribute positively to your quality goals.
Structuring Verification Activities
Verification activities should be structured methodically. At ISMS.online, we recommend:
- Defining clear objectives: Establish what you aim to achieve with each verification activity.
- Developing a verification plan: Outline the methods and frequency of verification to ensure ongoing compliance.
The Role of Verification in External Process Control
Verification acts as a safeguard, ensuring that:
- External processes are compliant: They meet your organisation’s quality standards and requirements.
- Continuous improvement is possible: By identifying areas for enhancement in external provisions.
Ensuring External Provisions Meet Requirements
To ensure external provisions meet specified requirements, you should:
- Conduct regular audits: Evaluate external providers to ensure they adhere to agreed-upon standards.
- Review supplier performance: Assess whether the external provisions align with your QMS objectives.
Tools and Templates for Streamlining Verification
utilising tools and templates can greatly streamline verification activities. We at ISMS.online provide:
- Audit checklists: To ensure all aspects of verification are covered.
- Templates: For consistent reporting and assessment of external providers.
By following these guidelines, you can ensure that your verification activities are both effective and efficient, contributing to the robustness of your QMS.
Documenting and Monitoring Supplier Selection Criteria
In the realm of Quality Management Systems (QMS), the meticulous documentation and monitoring of supplier selection criteria are paramount. As you navigate ISO 9001:2015 Clause 8.4, understanding and implementing these practices is crucial for ensuring the quality of your externally provided processes, products, and services.
Best Practices for Documenting Supplier Selection Criteria
To establish a robust supplier selection process, we at ISMS.online advocate for:
- Defining clear selection criteria: This should reflect your organisation’s quality objectives and customer requirements.
- Creating a standardised evaluation form: To ensure consistency and objectivity in supplier assessment.
Monitoring and Re-Evaluating Supplier Performance
Continuous monitoring is not just a requirement; it’s a strategic approach to quality management. You should:
- Conduct regular performance reviews: To assess if suppliers continue to meet your QMS standards.
- Implement a scoring system: For quantifiable and transparent evaluation of supplier performance.
The Necessity of Continuous Monitoring
Continuous monitoring is essential because it:
- Ensures ongoing compliance: With both ISO standards and your internal quality benchmarks.
- Facilitates proactive improvements: Allowing for timely adjustments in your supplier management strategies.
ISMS.online’s Support in Supplier Management
Our platform enhances your supplier management by providing:
- Integrated tools: For documenting, monitoring, and re-evaluating suppliers.
- Automated alerts: To remind you of upcoming evaluations and reviews.
By leveraging ISMS.online, you can ensure that your supplier management processes are not only compliant but also contribute to the continuous improvement of your QMS.
Manage all your compliance in one place
ISMS.online supports over 100 standards
and regulations, giving you a single
platform for all your compliance needs.
Communication of Requirements to External Providers
Effective communication with external providers is a cornerstone of a robust Quality Management System (QMS). It ensures that all externally provided processes, products, and services align with your organisation’s quality standards and objectives.
Conveying Product and Service Requirements
To effectively communicate your product and service requirements to suppliers, it’s essential to:
- Develop clear documentation: This should include specifications, drawings, and standards that suppliers need to meet.
- Hold briefing sessions: Where possible, discuss requirements directly with suppliers to clarify expectations and answer questions.
Key Elements of QMS Interactions
When interacting with external providers, ensure that the following key elements of your QMS are clearly communicated:
- Quality objectives: Suppliers must understand how their products or services fit into your quality goals.
- Process requirements: Detail the processes that suppliers must follow, including any necessary documentation or reporting.
The Cruciality of External Provider Competency
The competency of external providers is crucial because:
- It affects the quality of the final product/service: Incompetent suppliers can lead to substandard outputs.
- It impacts customer satisfaction: Ultimately, the quality of what you procure affects your customers’ experience.
Enhancing Communication with ISMS.online
At ISMS.online, we provide tools to enhance communication with your suppliers, such as:
- Collaborative platforms: For sharing documents and feedback in real-time.
- Training modules: To ensure suppliers understand your QMS requirements.
By utilising our services, you can streamline communication and ensure that all external providers are fully informed and competent to meet your QMS standards.
Ensuring Conformity in ISO 9001
Ensuring that externally provided processes conform to your Quality Management System (QMS) is critical. It’s about safeguarding the quality and consistency of your products and services, which, in turn, protects your brand’s reputation and your customers’ trust.
Determining Controls for External Provisions
To manage external provisions effectively, you should:
- Establish clear criteria: Define what constitutes acceptable quality and performance from external providers.
- Implement monitoring mechanisms: Use regular assessments to ensure ongoing compliance with these criteria.
Maintaining QMS Control Over External Processes
Maintaining control over external processes requires:
- Integration into your QMS: External processes should be treated as an extension of your internal processes.
- Regular audits: Conduct audits to verify that external providers adhere to your QMS standards.
Defining Controls for External Provider Output
Defining controls for external provider output is important because:
- It ensures consistency: You can maintain a uniform quality standard across all products and services.
- It facilitates traceability: Should issues arise, you can quickly identify and address the source.
ISMS.online’s Role in Inspection Protocols
At ISMS.online, we assist you in establishing robust inspection protocols by providing:
- Customizable checklists: To ensure all aspects of external provider output are inspected thoroughly.
- Documentation tools: For recording inspection results and maintaining an audit trail.
By leveraging our platform, you can ensure that your external processes are not just compliant, but also contribute to the excellence of your QMS.
Compliance doesn't have to be complicated.
We've done the hard work for you, giving you an 81% Headstart from the moment you log on.
All you have to do is fill in the blanks.
Assessing External Provider’s Control
In the context of ISO 9001:2015, Clause 8.4, assessing the effectiveness of an external provider’s controls is a critical step in ensuring that your supply chain contributes positively to your Quality Management System (QMS).
Methods for Assessing Control Effectiveness
To assess the effectiveness of an external provider’s controls, you should:
- Conduct audits: Regular audits can provide direct insight into the external provider’s processes and their alignment with your QMS requirements.
- Review performance data: analyse the provider’s performance metrics against the agreed-upon standards and objectives.
Verification Activities for Requirements fulfilment
Verification activities are essential to confirm that all requirements are being met. These activities may include:
- Inspections: On-site checks to verify that products and services meet your specifications.
- Testing: Assessing samples to ensure they meet the required quality standards.
Considering the Impact on Customer and Regulatory Requirements
Understanding the impact of external provisions on customer satisfaction and regulatory compliance is vital. Consider:
- Customer feedback: Monitor customer satisfaction to gauge the quality of externally provided products and services.
- Regulatory compliance: Ensure that external providers adhere to all relevant laws and regulations.
Leveraging ISMS.online’s Dynamic Risk Management Tools
At ISMS.online, we offer dynamic risk management tools that can aid in this assessment by:
- Identifying potential risks: Highlight areas where external providers might not meet your QMS standards.
- Tracking improvements: Monitor the effectiveness of any corrective actions taken by external providers.
By utilising these tools, you can maintain a high level of control over your external providers and ensure they meet your organisation’s stringent quality requirements.
Further Reading
Approval Processes for External Products and Services
Navigating the complexities of ISO 9001:2015, particularly Clause 8.4, requires a clear understanding of the approval processes for external products, services, and methods. This is where our platform, ISMS.online, can provide invaluable support.
Communicating Approval Requirements
Effective communication of approval requirements to external providers is essential. You should ensure that:
- Specifications are detailed: Clearly outline the quality standards and performance criteria expected.
- Approval criteria are transparent: Make sure external providers understand the benchmarks for acceptance.
Importance of Competence and Qualification
The competence and qualification of individuals involved in the provision of external products and services are critical because:
- Quality assurance depends on it: The skills and knowledge of these individuals directly impact the quality of the output.
- It affects compliance: Qualified personnel are more likely to understand and adhere to regulatory requirements.
ISMS.online’s Support in Approval and Release
At ISMS.online, we facilitate the approval and release process by providing:
- Structured workflows: To guide you through each step of the approval process.
- Documentation templates: To help you communicate requirements and record approvals effectively.
By utilising our services, you can streamline the approval process, ensuring that all externally provided products and services meet your stringent quality and compliance standards.
Monitoring and Control of External Providers’ Performance
Ensuring that external providers meet your organisation’s quality standards is a continuous process. At ISMS.online, we understand the importance of having a robust system in place to monitor and control the performance of your suppliers.
Methods for Performance Control and Monitoring
To maintain a high level of performance from your external providers, consider implementing the following methods:
- Performance metrics: Establish key performance indicators (KPIs) that align with your quality objectives.
- Regular audits: Schedule periodic audits to assess compliance with your QMS standards.
- Supplier scorecards: Use scorecards to provide a quantitative measure of supplier performance over time.
Verification and Validation at External Premises
Verification and validation activities are crucial for ensuring that your external providers are meeting your requirements. These can include:
- On-site inspections: Conducting regular visits to assess the provider’s processes and outputs.
- Sample testing: Evaluating samples from the provider to ensure they meet your specifications.
Documenting Evidence of Control Implementation
Documenting evidence of control implementation is vital because it:
- Provides a record of compliance: It’s essential for demonstrating due diligence and adherence to ISO 9001:2015 standards.
- Facilitates continuous improvement: Documentation helps identify areas for improvement in supplier performance.
ISMS.online’s Integrated Compliance Framework
Our integrated compliance framework supports performance monitoring by offering:
- Automated tracking: Keep tabs on supplier performance with automated updates and alerts.
- Centralised documentation: Store all evidence of control implementation in one secure, accessible location.
By utilising our platform, you can ensure that the performance of your external providers is consistently aligned with your QMS objectives.
Integrated Management Systems and ISO 9001 Compliance
In the pursuit of ISO 9001:2015 compliance, the alignment with “ANNEX L” is a strategic facilitator. This framework harmonises various management system standards, allowing for a seamless integration of quality management processes.
“ANNEX L” Alignment Advantages
“ANNEX L” provides a common structure that simplifies the integration of multiple management systems, which can:
- Enhance consistency across different standards.
- Reduce duplication of documentation.
- Streamline the implementation and maintenance of integrated systems.
Benefits of ISMS.online for QMS Processes
Our integrated management system, ISMS.online, offers several benefits for your QMS processes:
- Centralised control: Manage all your QMS documentation in one place.
- Real-time collaboration: Engage with your team and suppliers directly within the platform.
Enhancing Compliance with Pre-configured QMS Solutions
We provide pre-configured QMS solutions that:
- Offer immediate progress upon system login.
- Are tailored to meet ISO 9001 requirements, facilitating a quicker path to certification.
ISMS.online’s App Integration and Policy Management
Our platform’s app integration and policy management tools contribute to a holistic approach by:
- Allowing for the use of over 5000 apps to enhance QMS processes.
- Providing robust documentation tools for quality management and ISO 9001 compliance.