Why Is NIS 2 Reshaping Research Security-and Why Does It Matter Now?
The landscape of research security is undergoing a fundamental transformation, not because security threats are new but because the rules of trust, funding, and accountability have irrevocably changed under NIS 2. For research organisations, large or small, compliance is shifting from annual, back-office paperwork to a continuous, evidence-rich practise that impacts daily decisions, leadership priorities, and ultimately, the very reputation that secures ongoing grants and subsidy streams.
The NIS 2 Directive marks a decisive pivot: documented controls must be visible, operational, and provable in real-time. More than a compliance checkbox, NIS 2 makes information security an operational prerequisite for accessing public funding, advancing international partnerships, and maintaining sector credibility. Grant-makers and funders now expect research partners to flash live “trust signals”-evidence of active, role-mapped controls, streamlined audit trails, and quick-response incident reporting-at any point, not just year-end. The only path to future research alliance and funding velocity is proving your security stance before you’re even asked.
When trust is measured in seconds, evidence must move at the speed of your mission.
Shortened reporting windows, board-level responsibility, and the scaling expectation of continuous auditability all point toward a new normal. No research organisation-whether embedded in a university, aligned to commercial partners, or operating as a non-profit-can afford “compliance as afterthought.” Instead, compliance becomes the backbone of institutional agility, competitive positioning, and grant-winning credibility.
For stakeholders, this shift isn’t a hassle, but a necessary evolution: research cannot lead without assurance, and assurance isn’t real without live, accessible proof.
How Does NIS 2 Define Which Research Entities Are in Scope-and Why Is This a Moving Target?
Determining whether your research organisation is covered by NIS 2 is not a one-time exercise-it’s a dynamic evaluation, shaped by operational realities rather than historic labels or sector myths. No longer is it enough to assert a “teaching-first” shield; entities engaged in funded research, collaborating across borders, or controlling research outputs now bear the obligations of NIS 2.
The test for inclusion looks at operational role and funding mechanism. If your organisation touches external grant money, manages deliverables, or stewards research data-regardless of official university charts or departmental labels-you are in scope. Cross-border research further complicates this: every EU member state interprets NIS 2 differently. Any multi-partner project must proactively map the compliance routes for every jurisdiction involved, not just the home country’s standard.
Scope drift doesn’t happen in a boardroom-it happens in the middle of an urgent project.
Failing to determine scope at the outset is the silent killer of funding continuity. Delayed mapping leads to frantic document collation-often failing to produce the “structured evidence” demanded by grantors and auditors. In multi-entity partnerships, the controlling party for research output will carry the regulatory weight-if not by intent, then by default.
A living compliance mesh replaces static declarations: every grant submission, project kickoff, and partnership agreement must explicitly define compliance responsibilities, evidence expectations, and reporting workflows for each country in play.
Snapshot Table: NIS 2 Scope in Research
| Project Trigger | Scope Update | Action Needed | Prove With |
|---|---|---|---|
| New EU grant application | Cross-border assessment | Map compliance for all participant countries | Role/ownership log, risk map |
| Commercial research partnership | Supplier liability exposure | Add supply chain control mapping | Supplier contract, comms log |
| Teaching-focused entity joins | Teaching only? (likely not all) | Verify funding/work output streams | Org chart, funding breakdown |
| Internal reshuffle | Scope drift | Re-assess assets, revise SoA | Updated SoA, org/risk review |
Start mapping compliance at project inception; retroactive efforts only create audit and funding pain.
Master NIS 2 without spreadsheet chaos
Centralise risk, incidents, suppliers, and evidence in one clean platform.
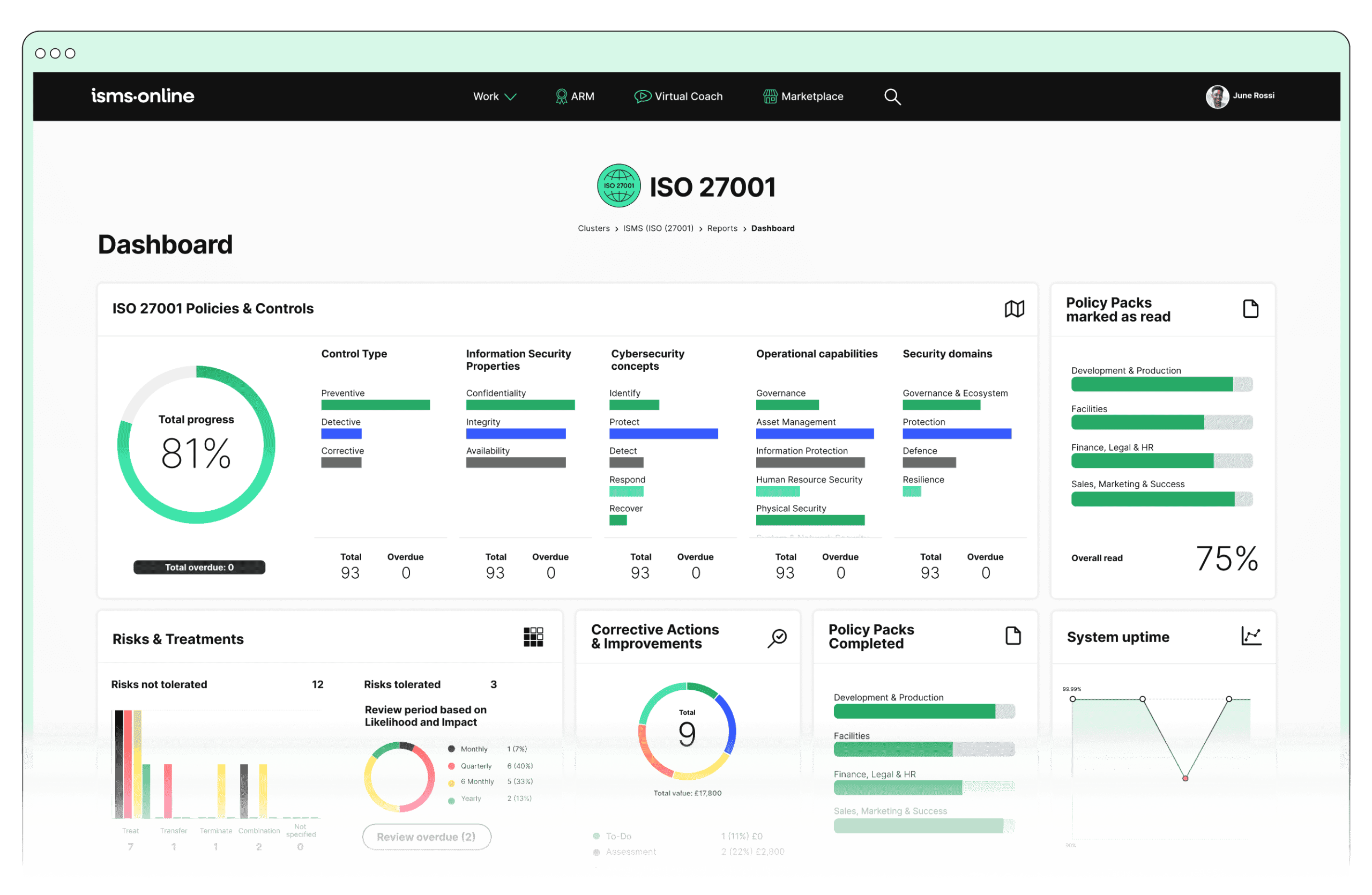
What Core Security Controls Does NIS 2 Now Require for Research Orgs?
NIS 2 is not a flavour of ISO 27001-it is a continuous operating system for research security, layering live, role-based controls and instant auditability onto your information management workflows. Controls are not optional or static; they must be operational, assigned by name, and provable across every stage.
Real-Time Accountability: Article 21 in Practise
Article 21 mandates operational controls for risk management, incident response, supply chain security, and ongoing evidence generation. Organisational diagrams, policy PDFs, and annual reviews are no longer sufficient-auditors now examine updated, timestamped logs, role assignments, and control execution as proof of compliance.
The new expectation: everything from onboarding a new board member to adding a supplier is mapped with documented controls, validated approvals, and instantly accessible logs.
A policy is no longer evidence; only live action logs show compliance.
ISO 27001 to NIS 2 Bridge Table
| Expectation | Operationalisation | ISO 27001 / Annex A Reference |
|---|---|---|
| Named owner for controls | Control/role matrix, workflow logs | A.5.2, A.5.4 |
| Supplier security | Due diligence, mapped risk | A.5.19, A.5.21, A.5.20 |
| Policy lifecycle | Versioned reviews & logs | A.5.1, A.5.36 |
| Role-specific evidence | Approval trails, assignments | SoA roles & SoA logs |
Practitioners must shift from isolated, checklist-based compliance to a continuous, versioned log system that ensures audit-proof evidence is always available-never a last-minute scramble.
What Does “Proving Compliance” Look Like Under NIS 2?
Old audit practises-compiling last-minute document bundles from file shares or trying to reconstruct decisions after the fact-are formally obsolete. NIS 2 recognises only centralised, continuously-updated, and role-tagged evidence.
An incident workflow is now a chain: collect, triage, notify, log, and review, with every step linked to responsible actors and timestamps. This structure means practitioners not only reduce their administrative overhead but are also less exposed to handoff failures and audit surprises.
A single missing timestamp can shift blame-and cost-onto your team.
Traceability Shortcuts: Risk → Action → Control Proof
| Trigger | Risk Update | Control / SoA Link | Evidence Logged |
|---|---|---|---|
| New risk emerges | Add/modify risk register | A.5.5, A.5.7, A.5.8 | Updated registry, SoA assignment |
| Incident (real or near-miss) | Capture & escalate process | A.5.24–A.5.26 | Incident log, staff comms |
| Supplier onboarding | Conduct risk & role assessment | A.5.19–A.5.21 | Supplier evidence, logs |
Centralisation and automation cut prep time for IT/security managers and compliance officers by more than half. For grant renewal and board assurance, nothing beats instant, on-demand export of well-tagged, living evidence.
Be NIS 2-ready from day one
Launch with a proven workspace and templates – just tailor, assign, and go.

How Do Risk Management and Incident Reporting Accelerate Under NIS 2?
NIS 2 slashes reporting latency: incidents-real or near-miss-must be reported within just 24 hours for initial notification and within 72 hours for full detail. Silence or sluggish reporting is now an operational and reputational risk.
“Manual collation” is extinct; only automated, role-specific logs demonstrate judgement and readiness. Everything from a phishing attempt to a supply chain interruption is now a logged step: reviewed, assigned, and acted upon, with automatic reminders and reporting triggers calibrating the organisational response.
Incident Evidence Automation
A live incident management platform links the following for every event:
- Time of incident
- Staff involved (by role)
- Notification chain
- Mitigation steps
- Post-incident review (lessons learned)
Versioned, accessible logs are now the only evidence that a process-not just a response-actually exists.
Proving compliance is now a live discipline-one that enables practitioners to move from after-the-fact explanations to proactive, defensible operations, trusted by funders and regulators alike.
Why Is Supply Chain Security Now the Acid Test for Research Orgs?
NIS 2 recognises the harsh reality: security is only as strong as the weakest supplier or partner in your chain. Every vendor, commercial tie, or collaborator now becomes an “inherited compliance risk”-making supplier management a proactive audit priority.
Operationalising Two-Layer Control
- Every supplier is risk-assessed before onboarding.
- Ongoing reviews update risk ratings, and any incidents linked to suppliers are logged and reported.
- Contract terms now mandate reciprocal reporting and mutual evidence of compliance readiness.
If your supplier can’t prove it, you can’t either-to the auditor, you’re responsible.
Evidence Map: Supply Chain Controls
| Trigger | Risk Update | Control / SoA Link | Evidence Logged |
|---|---|---|---|
| New provider engaged | Supply chain risk mapping | A.5.19–A.5.21 | Due diligence log, contract |
| Supplier event (incident) | Supplier notification & impact | Incident response, legal | Notification log, assessment |
| Recurring review | Ongoing risk update | Risk / supplier review | Meeting notes, renewed contract |
Centralised, platform-based evidence mesh ensures supply chain security is not just a spreadsheet checklist but a living, adaptive, two-layer proof of due diligence-exportable for any stakeholder.
All your NIS 2, all in one place
From Articles 20–23 to audit plans – run and prove compliance, end-to-end.

What Happens When National Security or Dual-Use Research Is Involved?
National security and “dual-use” (civil and defence) research projects automatically trigger stricter controls and heightened oversight. Every step, from project inception to international collaboration, is taggable, traceable, and subject to documentation and export controls that can trigger funding freezes, partnership suspensions, or even sanctions if mishandled.
High-risk projects:
- Mandate appointed board/authority oversight.
- Require tagged logs for access control, incident records, and export screening.
- Must document and regularise training/upskilling, authority sign-off, and regulator notifications.
What used to be a hidden label is now a central pillar of compliance evidence.
Falling short on dual-use governance isn’t simply a paperwork gap-it’s an existential threat to project continuity and public/private funding reliability.
How Do You Build a Continuous Compliance Mesh-And What Is the Real-World Payoff?
A modern research organisation earns its credibility by weaving together every process, control, and risk into a unified compliance mesh-removing friction for the team and doubt for the auditor or funder. Scattered logs, hand-built spreadsheets, and siloed folders invite error and delay; centralised versioned logs, continuous role-mapping, and auto-export capabilities clear paths for fast renewal, trustworthy partnerships, and operational confidence (isms.online).
Within ISMS.online, this mesh means:
- Every control and risk is mapped to a real actor and a live versioned log.
- Every incident and update is export-ready for any audit scenario.
- Supply chain and dual-use risks are embedded, not appended.
- Compliance is integrated directly into your operational cycle-training, HR, finance, and legal have a stake, not just IT.
Operational compliance isn’t a report; it’s a permanent state of readiness.
Traceability Example Table: From Risk to Evidence
| Trigger | Risk Update | SoA/Control Link | Evidence Logged |
|---|---|---|---|
| New AI project | Algorithmic supply chain risk | SoA mapping, NDAs | Project log, controls list |
| Funder evidence request | Compliance mesh export | Audit pack/history | PDF export, stakeholder map |
| Board audit dry run | Role/approval review | Management review cycle | Board minutes, logs |
Ready access to this “evidence mesh” has a hard ROI: fewer failed audits, faster grant renewal cycles, and visible readiness-turning bureaucratic overhead into funding advantage.
What Does Real, Stakeholder-Ready Compliance Look Like-And How Can You Achieve It Today?
Stakeholder-ready compliance means every part of your organisation-project managers, researchers, IT, finance, and board members-can see, export, and explain security status instantly. For researchers and grant-seekers, this translates to smoother funding flows; for practitioners and leaders, it offers real protection against staff burnout, audit failure, and reputational damage.
ISMS.online enables this by centralising and automating the living compliance mesh: role-tagged controls, automated logging, versioned policy packs, audit-ready exports-all in one place, all the time.
Compliance, lived and proven, is the new baseline for research alliances and funding.
The payoff is not just auditor praise, but the real shift from survival mode to advantage: audit reports delivered at speed, grant renewals frictionless, stakeholder trust proven not with promises but with live, ready evidence.
Own Your Research Organisation’s Compliance Destiny
NIS 2 is here, but compliance is not a finish line-it is a mesh, woven moment by moment, connecting policies, evidence, and people to every audit, every partnership, and every funding milestone. Platforms like ISMS.online put this in reach, not just for large research institutions, but for every entity ready to move compliance from hindrance to habit, from bottleneck to badge of leadership.
Your next audit requirement is today’s ticket to funding. Make it as easy, exportable, and defendable as possible. Invite your team and leadership to see a living compliance mesh in action-because resilience, earned daily, is your competitive edge and contribution to research that truly matters.
Frequently Asked Questions
Why do NIS 2 obligations pose unique challenges for research organisations?
NIS 2 redefines cyber-security for research organisations by imposing continuous, real-time oversight-far beyond the episodic, “once a year” audits familiar from ISO 27001. Now, every research project and collaboration, regardless of timing or funding, must be supported by live, version-controlled evidence that is always audit-ready. For fast-moving labs and consortia juggling sensitive data, shifting teams, and funding deadlines, this creates friction at every level: academic exemptions no longer provide cover, responsibility for compliance is both centralised at the board and diffused across project teams, and documentation gaps risk not only penalties but lost grants and reputational harm.
In research, yesterday’s slow compliance is today’s greatest liability-delayed records now threaten both science and funding.
Unlike commercial entities with stable procedures, research groups often experience role churn, partner flux, and project pivots. NIS 2 holds the board legally accountable for failures but leaves no room for missing logs or ambiguous roles; evidence must be traceable, tied to named individuals, and responsive within days or hours, not months. Centralising controls and automating role-based evidence collection-with platforms like ISMS.online-turns compliance from just another administrative load into a pathway for securing new grants and building stakeholder trust.
Fresh NIS 2 Realities for Research Groups
- Live, continuous compliance: Every control, log, or incident must be retrievable and time-stamped on demand.
- Role clarity and chain of custody: Each project action, policy change, or incident is mapped to a real person, not a generic group.
- Board-level liability: Directors are now just as exposed as IT for missing evidence or delays, incentivizing system-wide compliance culture.
How can research organisations know if they fall under NIS 2 – and what’s changing with national versus EU law?
Determining NIS 2 scope is anything but static. If your research group handles sensitive data, accepts EU or national grants, builds prototypes with third parties, or contributes to projects tied to critical infrastructure or cross-border impact, you’re likely in-scope by default-even if the university had exemptions before. National implementations can diverge rapidly: regulations and research sector guidance shift with new legal interpretations, often extending obligations to previously exempt pure academic or non-profit entities. Each new project, international collaborator, or funding cycle should trigger a reassessment-especially since national authorities can move the compliance “goal posts” with little notice. Most critically, audit documentation must show not just whether you checked the rules once, but whether you’re tracking scope and role assignments at every major change.
Quick Scope Assessment Matrix
| Trigger | Legal Review Required? | Evidence to Update | Responsible Owner |
|---|---|---|---|
| New EU or national grant | Yes | Scope log, project registry | Project Lead, Legal |
| Change in project partners | Yes | Consortia registry, SOW | Board, Compliance |
| Pivot to commercial or critical sector | Yes | Risk register, policy update | Exec, PM, DPO |
The only defence against scope drift and last-minute audit surprises is persistent, logged visibility into your obligations.
What concrete security controls and audit evidence does NIS 2 demand from research teams?
NIS 2 turns every security control into a live, auditable process. It requires not just policies and access lists, but granular, versioned logs showing who changed what, when, and why-linking each action to actual people, systems, and outputs. For risk management, incident response, and supply chain, NIS 2 expects clear assignments (e.g., “Security Lead,” “Data Protection Officer”), evidence tied to project milestones, and robust mapping to ISO 27001 and ENISA standards. Audit-readiness means answering questions like, “Show every change to our encryption protocol, by whom and when”; “Export all supplier risk reviews for the last 18 months”; “Where was the last critical incident handled, and who signed it off?”
NIS 2-ISO 27001 Compliance Reference Table
| NIS 2 Area | Evidence Type | ISO 27001 Point | Responsible Role |
|---|---|---|---|
| Information Security Policy | Versioned, signed policy | A.5.1, A.5.36 | Security Lead / DPO |
| Supply Chain Assurance | Annual supplier audit | A.5.21 | Procurement/PM |
| Incident Response | Timestamped incident log | A.5.24–A.5.26 | CSIRT/IT Management |
Auditability is only achieved by maintaining a centralised digital “control room”-not ad hoc spreadsheets. For research, auditability is now both a compliance requirement and the competitive proof needed to secure high-value grants and cross-border partnerships.
How does NIS 2 operationalise risk and incident management for research organisations?
NIS 2 elevates risk and incident management from static compliance exercises to dynamic, real-time workflows. Every risk-be it a technical vulnerability, staff change, supply chain gap, or even a failed phishing attempt-must be continuously assessed, triaged, and version-logged from identification to closure, with outcomes routinely escalated to the board or compliance leadership. Incidents are on the clock: major events may require notification to authorities within 24 hours, followed by a root-cause analysis and remediation proof within 72 hours, all linked back to the relevant controls and asset registers. Critically for research, even “near misses” and small disruptions that could impact public services, privacy, or grant obligations must be catalogued and reviewed-waiting until year-end is not just risky, it’s non-compliant.
Key Audit-Ready Actions
| Event | Time to Notify/Report | Required Evidence | Accountable Role |
|---|---|---|---|
| Major Incident | 24h warning to regulator | Incident log snapshot | CSIRT / Security |
| Full Review | 72h after event | Root cause, remediation log | DPO / Risk Manager |
| Closure | Within 1 month | Lessons learned, audit export | Board / PM |
Tracking every event, not just the big ones, is now both protection and a grant-winning differentiator for research organisations.
Why is supply chain security a central concern for NIS 2 compliance in research?
Your research organisation’s security is now inherently tied to the risk posture of every supplier, partner lab, or contracted specialist-NIS 2 makes no distinction between internal and third-party controls. Any partner, software provider, or vendor with access to research systems or data must be risk-assessed before onboarding, contractually bound to notify you of incidents, and subject to routine compliance checks or certifications. Annual “set and forget” diligence is not enough: auditors and funders expect to see live logs of ongoing supplier/end-user risk status, registry updates for even minor incidents, and contractual evidence of reciprocal obligations.
Central Supply Chain Security Workflows
- Initial onboarding: Conduct formal risk mapping, store evidence in a central log, and require signed compliance commitments.
- Ongoing evidence: Annual or scheduled supplier reviews, renewed certifications, and live update trails for any partner changes.
- Responsive compliance: Immediate documentation and registry alerting for supplier incidents or status changes.
| Supply Chain Trigger | Compliance Step | Responsible Party |
|---|---|---|
| New partner onboarded | Risk-map, formal log update | PM / Procurement |
| Supplier incident | Log, notify, update contracts | Security / Compliance |
| Routine audit | Registry review, board notify | Board / Sec Lead |
Supply chain instances are now the quickest route to non-compliance or funding risk-live, reciprocal monitoring isn’t optional.
How do national security and dual-use requirements impact NIS 2 compliance for research?
If your organisation’s research touches on dual-use technologies, national security, or critical infrastructure, NIS 2 supervision and penalty risk increases exponentially. Projects must be tagged on intake for critical or dual-use relevance, tracked in segregated compliance packs, and have their evidence logs managed with the same rigour as IT infrastructure-this includes versioned access records, export screening, and regulator engagement. Any missed protocol in these projects-failure to log a transfer, unclear approval roles, skipped incident review-can result in suspended grants or project shutdown, not just fines. Legal and compliance leads must monitor these projects continuously, and board signoff becomes mandatory before key decisions, access changes, or technology transfers occur.
Dual-Use/High-Trust Research Compliance Path
- Early tagging and alerting: Prompt legal review and intake in a separate evidence pack.
- Secure documentation: Version, role-map, and time-stamp every relevant activity and access.
- Regulator coordination: Maintain readiness for pre-review or urgent evidence demands.
Compliance is existential in dual-use scientific domains: regulatory failure can bring entire research programmes to an immediate halt.
What marks out “effective” NIS 2 compliance for research labs in 2024 and beyond?
Effective NIS 2 compliance is now defined by cross-functional, “living” systems-where every project, policy, control, incident, asset, and supply chain partner is tracked, mapped, and instantly exportable. Modern research organisations are underpinning their compliance with digital meshes: unified controls linked across NIS 2, ISO 27001, and ENISA benchmarks; real-time dashboards for risks, incidents, and supplier health; automated reminders for evidence renewal, expiry, staff roles, and incident triage. Critically, the compliance mesh reaches every corner-IT, HR, legal, procurement, security, and the board-making compliance a daily operational feature, not an annual afterthought.
Features of a Living NIS 2 Compliance Mesh
| Mesh Feature | Demonstrable Outcome | Platform Capability |
|---|---|---|
| Unified mapping | No control gaps or duplications | ISMS, risk, asset mapping |
| Live role dashboards | Board/partner/funder trust, quick audits | Automated, versioned logs |
| Automated workflow | Zero missed renewals or deadline lapses | To-dos, reminders, expiry |
| Export-on-demand | Auditable, regulator/funder proof | Instant evidence export |
Show your funders and partners you’re not just compliant-you’re resilient and audit-ready. With ISMS.online automating your evidence, mapping every obligation, and empowering all contributors, your research compliance becomes both your shield and your passport to new collaborations, funding, and impact.