Why Has NIS 2 Redefined Research as Critical Infrastructure?
Europe’s research sector has undergone a drastic reclassification. Under NIS 2, universities, public and private research institutes, medical networks, and scientific consortia now share the same regulatory battlefield as energy grids and national banks. The logic is simple and sobering: modern science doesn’t just generate knowledge-it underpins whole economies, national security, and vital services. Research is no longer off to the side of critical infrastructure; it is critical infrastructure. If a data breach stalls a lab’s workflow, delays a lifesaving project, or leaks patient data across borders, the entire sector pays.
The NIS 2 Directive came in response to a sobering pattern-a succession of ransomware attacks, intellectual property thefts, and supply chain breaches within Europe’s research community. What began as “IT’s problem” is now every director’s daily risk-and a leadership duty. Regulators have ratcheted up not just penalties, but direct accountability: lack of compliance can disrupt cross-border funding, partnerships, and eligibility for new grants.
The new reality: cyber risk defines whether your research team is seen as credible, resilient, and fundable.
The reasoning underpinning NIS 2 is clear: a single breach in a university or research consortia can trigger project shutdowns, endanger ongoing EU-funded work, damage reputations, and even risk supply chains that European businesses and governments rely on. If science is society’s engine, cyber threats are potholes that can snap axles: tomorrow’s grants, partnerships, and even sovereignty are on the line.
How Far Does NIS 2’s Scope Extend-and Who Must Take Notice?
No institution wants a surprise audit or notification, yet NIS 2’s reach is remarkably wide-by design. Unlike sector-centric laws of the past, NIS 2 zeroes in on any operation, public or private, whose research activities tie into national interest, critical infrastructure, or pan-European project funding. Its tentacles stretch well beyond household-name universities.
Understanding Operational Scope and Rare Exemptions
- Large universities and national centres: Almost always in scope, with few exceptions.
- Specialist research teams and SMEs: Not exempt by default. If you supply unique datasets, manage critical research equipment, or process grant administration for pan-EU projects, compliance is a likely requirement.
- International/European funding involvement: Virtually guarantees entry into the compliance loop, even for smaller institutions.
- Public vs. private: Legal status seldom matters; actual operations, scale, and criticality do. Exemptions exist, but only through explicit designation and are rare.
To make things trickier, individual member states have the leeway to tweak exactly where the lines fall, but the conservative assumption is clear: unless formally told otherwise by a designated regulator, your research unit is in scope. If you delay or misclassify, you don’t just invite enforcement-you become a liability for future funders and collaborators.
One delayed notification or incomplete classification can erode trust instantly-funders move on.
Visual cue: Picture a branching decision map-regardless of status, size, or funding model, most paths twist back to ‘in scope until proven otherwise’.
Master NIS 2 without spreadsheet chaos
Centralise risk, incidents, suppliers, and evidence in one clean platform.
Who Answers When Collaboration Goes Wrong? The Accountability Web in Research Networks
Research isn’t a solo game. Pan-European science, pharma, clinical research, climate modelling-the projects that matter are multi-team, multi-system, and often multi-country. NIS 2 dramatically raises the stakes for these consortia.
Shared Responsibility-and Mutual Risk
- Cross-border consortia and supply chains: Every partner-lead institutions, principal investigators, tech providers, or data hosts-shares responsibility for reporting incidents, remediating vulnerabilities, and sustaining compliance.
- Incident in one, consequences for all: A single breach involving a shared platform or dataset requires rapid, documented reporting from every partner-not just those who “own” the system.
- Partnership agreements: Must spell out not just duties but *evidence requirements*. “Intentions” or informal policies no longer cut it.
- Documentation and review: Auditors expect logged lists of contacts, evidence of regular workflow reviews, and supply chain escalation trees.
In NIS 2, a compliance blind spot at any joint-touchpoint is everyone’s liability.
Visual cue: A research network map linked by arrows, each connecting to reporting flows, supply chain notifications, and audit artefact logs-demonstrating how a single incident radiates across the system.
What Are the Absolute Essentials for Research Compliance Leadership?
Under NIS 2, success means turning compliance from a passive audit risk into a living, breathing part of research management. Leaders and compliance heads must prioritise the controls that directly move the needle-on both regulation and operational resilience.
Four Non-Negotiable Controls
-
Rapid, Documented Incident Reporting
Notifying relevant agencies within 24 hours is table stakes. Full incident reporting by 72 hours is mandatory. This isn’t just IT’s job-executive and board-level signoffs are required. -
Live Risk Register & Board-Signed Policy Suite
The age of static policy binders is over: risk registers and policy libraries must be mapped, versioned, and reflect reviewed decisions. Boards must review and sign off annually. -
End-to-End Supply Chain Control
Every partner, cloud vendor, and research supplier is under ongoing, documented scrutiny. Surveys at onboarding are not enough; proof of consistent review is required. -
Staff Training & Simulation Evidence
Annual cyber awareness and role-specific incident simulations must be logged and verifiable. It’s no use claiming “we train everyone” without showing participation, outcomes, and evidence of improving gaps.
Without active, operational records, policies remain invisible to auditors-and to consortia, regulators, or prospective funders.
Audit Differentiators
Grant and funding committees are already selecting future partners using criteria like:
- Versioned, board-signed policies mapped to live, current risk registers.
- Evidence of simulation, training, and correction action logs attached to real-world incidents.
Be NIS 2-ready from day one
Launch with a proven workspace and templates – just tailor, assign, and go.

Who Holds the Line on Compliance? Governance, Fines, and Management Responsibility
NIS 2 ends ambiguity around who is ultimately liable. The spotlight is firmly on senior management, directors, and those with operational oversight.
Governance Duties (and Personal Risk)
- C-suite and board-level accountability: Direct legal liability for failure to resource, monitor, or sign-off on system-wide cyber controls.
- Proof of engagement: Auditors need more than paper policies; logs must show active participation in management reviews, training, and policy signoff meetings.
- Fines, enforcement, and reputational penalties: Penalties extend into the millions or a share of turnover-comparable to GDPR. Individuals can face liability, especially in public and non-profit research bodies.
Board-level action is now a compliance artefact-a signed annual incident response plan and minutes from management meetings may one day be your only legal defence.
Management and Board Actions
- Maintain an up-to-date incident response and escalation matrix.
- Log every substantive review of security policy, audit result, and action or decision in formal, time-stamped records.
- Monitor, review, and demonstrate resource allocation to compliance and security standards.
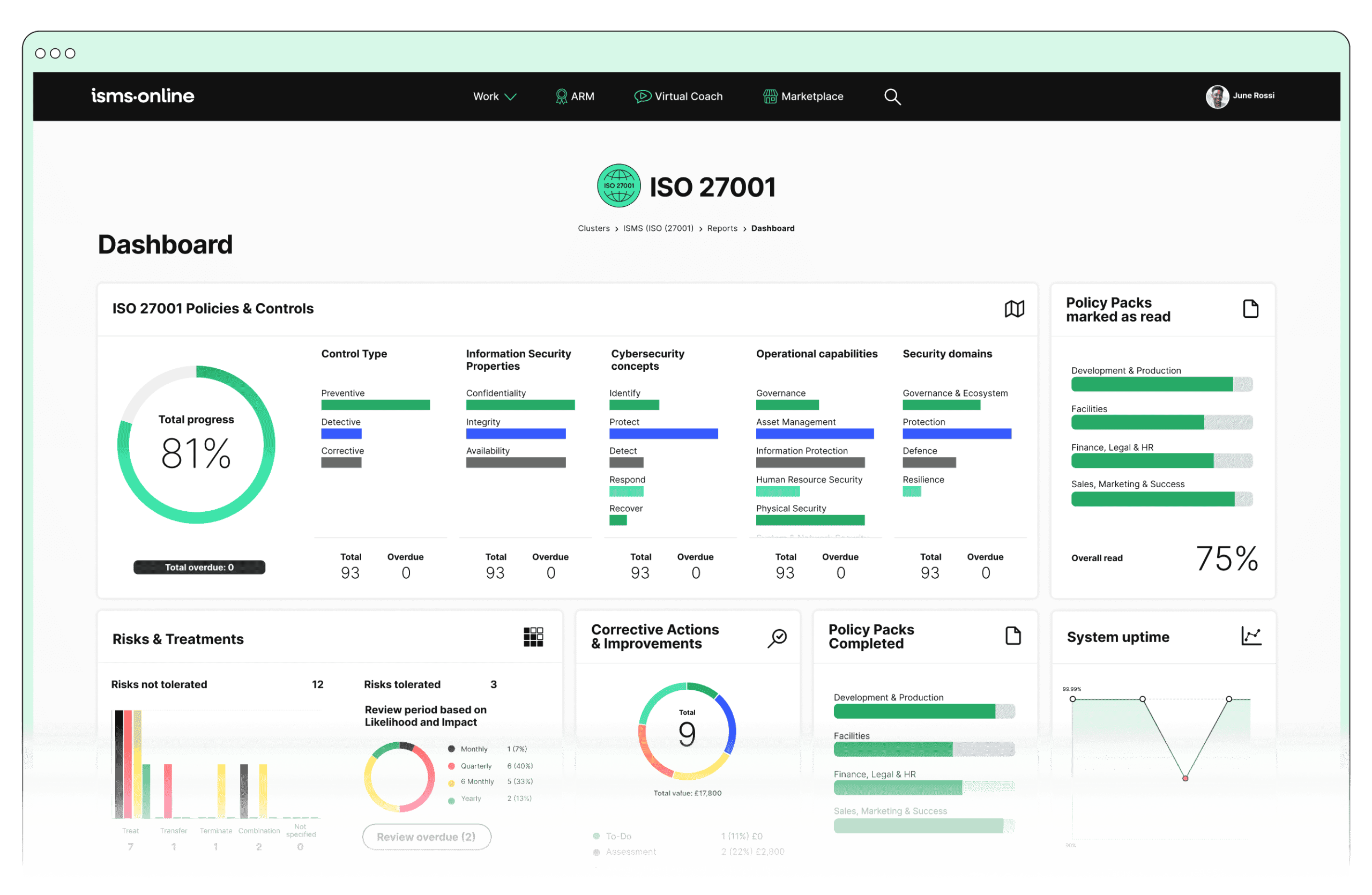
How Do the Requirements of NIS 2 Map to ISO 27001? Gaps, Integration, and Best-In-Class Moves
ISO 27001 remains the gold standard for research sector information security-but NIS 2 carves out higher policy, reporting, and supply chain obligations. For most, an “ISO 27001-first” strategy covers the foundation, but auditing, executive engagement, and real-time supply chain monitoring are new frontiers.
ISO 27001 / NIS 2 Bridge Table
| **Expectation** | **Operationalisation** | **ISO 27001 / Annex A Ref.** |
|---|---|---|
| 24/72hr incident response | Incident playbooks/comms | A.5.24, A.5.25, A.5.26 |
| Supply chain vigilance | 3rd party due diligence | A.5.20, A.5.21, A.5.22 |
| Board-approved security policy | Annual management review | Clause 5.2, A.5.1, A.5.4 |
| Risk register updating | Regular scheduled reviews/logs | Clause 6.1, 8.2, A.5.7, A.5.35 |
| Board/staff training logging | Acknowledgement logs, attendance | A.6.3, A.5.36 |
| Central evidence management | Time-stamped audit logs | Clause 7.5, 9.1, A.5.35, A.5.36 |
While ISO 27001 is foundational, research entities must surface continuous board engagement, live supply chain monitoring, and prompt incident response as additional compliance and operational priorities.
All your NIS 2, all in one place
From Articles 20–23 to audit plans – run and prove compliance, end-to-end.

What Does “Auditor-Ready” Look Like for a Research Sector Entity?
Modern compliance teams recognise that it’s not just about having documentary evidence, but ensuring live, actionable traceability across the entire compliance lifecycle.
Move Beyond Spreadsheets-Go Platform-Driven
- Central platform: Ditch disconnected file-shares and manual folders. Purpose-built compliance platforms centralise evidence, automate policy updates, and keep staff acknowledgements up-to-date.
- Live document logs: Real-time audit dashboards track every risk update, control status, and board decision.
Traceability Table (The Compliance Loop in Action)
| **Trigger** | **Risk Update** | **Control/SoA Link** | **Evidence Logged** |
|---|---|---|---|
| Supplier breach | Entry in risk register | A.5.21 | Incident + Third-party contract |
| Revised national rule | Update policy wording | A.5.1 | Signed policy + review log |
| Simulated incident | Update protocol | A.6.3 | Simulation log + attendance record |
| Audit finding | Review/correct | A.5.35, A.5.36 | Audit log + corrective record |
Visual cue: A compliance dashboard showing real-time matching of triggers to risk register entries, controls, and evidence-surfaced for every team and auditor.
The fastest-growing research teams use platform-driven evidence to power pass-first-time audits-and gain an edge with funding partners.
What Should a Proactive Research Team Do Next to Achieve-and Show-NIS 2 Compliance?
1 priority: integrate compliance and resilience as part of your operational DNA, not as a report filed after the fact. Here’s where sector leaders are pulling ahead.
Proactive Organisation Steps (and the Cost of Delay)
- Regular incident rehearsals: Teams meet on a monthly cadence, stage simulations, and review outcomes across IT, legal, HR, and grant management.
- Matrixed compliance leadership: Compliance is led not just by IT, but by a team combining legal, HR, grants, and IT, with a central evidence review calendar.
- Unified platform: Move evidence, risk, audit, and policy tasks into a single collaborative system, surfacing issues and reminders for management action.
Teams lagging behind face a triple threat: fines, exclusion from grant competitions, and reputational harm. Delays cost more than money-they stifle strategic partnerships and scientific impact.
Quick Wins to Build Momentum
- Deploy NIS 2–ready compliance platforms with pre-mapped template policies and workflows.
- Automate review calendars and dashboards to maintain staff engagement and surface risks.
- Start your team with a calibration audit to benchmark controls and risk register status.
Ideal Team Structure
- Compliance lead: with direct board reporting line.
- Matrix wings: IT/security, Legal, HR, Grants/Finance.
- Central collaboration dashboard: policy, risk, and audit status always visible.
Visual cue: Org structure radiating from compliance leadership to cross-functional teams, all reporting into a central platform.
Take the Advantage – Lead Your Sector with Operational Confidence
Where compliance was once a cost-centre, today it’s the signature of a research institution’s maturity, trustworthiness, and agility.
For Research Leaders
Bring your teams together for a live workflow demonstration-see what centralised evidence management looks like in action. Don’t just talk readiness: show rapid-launch incident and policy templates specifically tuned for the research sector. Invite funders and consortia partners to your next management review roundtable.
For Security, IT, and Privacy Practitioners
Try a compliance platform demo-walk through how evidence is versioned, audits tracked, and staff acknowledgements logged without endless spreadsheets. Explore template packs for SAR (Subject Access Requests), DPIA (Data Privacy Impact Assessments), and incident response logs, built for your workflows.
For Legal and Board
Demand a compliance mapping session: visualise exactly how your policies, risk registers, and documentation align to NIS 2 and ISO 27001. Use the outputs not just for self-assurance, but to win your next round of grant funding and strategic partnerships.
Institutions that move now win more than compliance-they lead in funding, reputation, and scientific progress.
Dont Wait-Make Compliance Your Research Advantage
Embed agility by keeping frameworks, registers, and evidence live and refreshable. Every improvement feeds your next audit-and your next funding or partnership opportunity. Start with an honest calibration: close compliance gaps, surface evidence automatically, and iterate faster than your competitors. NIS 2 isnt just a new burden-its your institutions chance to lead.
Book a demoFrequently Asked Questions
Who qualifies as an “in-scope” research organisation under NIS 2-and what real exceptions exist?
If your research organisation employs 50 or more staff or has an annual turnover above €10 million, you are almost certainly within NIS 2 scope-even more so if you engage in EU-funded initiatives, cross-border collaborations, or deliver research impacting critical sectors like health, energy, or infrastructure. This net covers most universities, independent institutes, not-for-profits, and public or hybrid research centres.
National authorities may extend coverage further based on local risk assessments or the strategic importance of your work; conversely, true exemptions are rare and depend on whether a disruption would pose no public or cross-sector risk. Older exclusions for “education” or “public bodies” are now largely obsolete-NIS 2 purposefully closes those legacy loopholes,.
Many assume If we’re a university or non-profit, we're out of scope. That’s now the exception, not the rule.
How to confirm your status:
- Check your headcount and turnover against thresholds, but also scrutinise funding streams and project partners-public grants and infrastructure research may trigger “critical” status.
- Review your country’s published lists of essential/important entities; some Member States broaden NIS 2’s net even further.
- When uncertain, request clarity in writing from your sector regulator or CSIRT, as “grey zone” roles (spinouts, joint ventures, digital/AI labs) often trigger discussions.
What are the essential NIS 2 compliance requirements for research organisations?
NIS 2 demands more than paper policies: it mandates demonstrable, board-owned security maturity in five domains:
- Risk Management: Map and review every critical digital asset, process, and supplier. Maintain a living risk register-don’t wait for annual cycles. Document threat scenarios like ransomware, third-party breach, or system outage.
- Governance & Policy Oversight: Your board or appointed directors must visibly own and review security policies, risk status, and incident plans at least annually, signing off with traceable minutes.
- Documentation & Audit Trails: Keep full version histories for every policy, risk update, staff training, and external assessment. Ensure “who changed what, when, and why” is provable.
- Supply Chain Security: Vet all key suppliers and partners, document security expectations in contracts, schedule periodic supplier reviews, and record incidents linked to vendors.
- Incident Response & Reporting: Be ready to escalate and report incidents within 24-72 hours, run playbook rehearsals, and log after-action learning. Responses must reach local/national authorities, funders, and project partners.
Under NIS 2, senior leaders and directors face personal liability for failures to resource, review, or enforce these areas, TÜV SÜD Guide).
How can your organisation embed NIS 2 compliance into daily operations, not just annual review?
Treat compliance as a continuous discipline, not an annual project. Start by calibrating your current posture-benchmark risk registers, incident processes, and policy reviews using an ISO 27001-aligned platform if possible.
Practical steps to operationalise compliance:
- Appoint direct board ownership: assign a named leader as security and incident escalation authority.
- Centralise records: Use a compliance platform to unite policy, risk, incident, and supplier documentation-spreadsheets scatter evidence and slow responses.
- Run monthly or quarterly workshops to rehearse playbooks, walk through supplier reviews, and red-team reporting scenarios. Simulate 24-hour and 72-hour notification drills to test readiness.
- Log every cross-department improvement-show auditors a living improvement loop, not just static policy.
- Embed compliance logging and engagement into finance, legal, HR, and research management workflows.
A visible, living compliance dashboard plays as much for future funding and partnership trust as it does for surviving audits.
What are the real consequences-and personal risks-for directors if a research entity fails NIS 2?
NIS 2 empowers authorities to impose fines up to €7 million or 1.4% of annual worldwide turnover, whichever is higher. More crucially, directors, trustees, and senior managers may be personally named in enforcement actions if there’s evidence of poor oversight, missing board review minutes, unremedied risk logs, or under-resourced security programmes,.
But the business consequences bite harder:
- Funding risk: Ineligibility for grants, EU calls, or major tenders; funders now expect structured policy logs and improvement trails before any award.
- Consortium reputation: Partners increasingly vet compliance up front and may drop non-compliant members.
- Public trust: Regulator actions or negative publicity can cost more than fines-harming stakeholder, student, and board confidence.
- Career impact: Absence of process logs, risk updates, or clear audit trails can be interpreted as personal negligence, with real career ramifications.
Regular management review logs and improvement actions are your costly signals of diligence-neglect becomes visible and actionable.
What separates “audit-ready” NIS 2 organisations from those at risk?
To be audit-ready, your organisation needs real-time, structured evidence mapped directly from boardrooms to operational trenches:
Key documentation attributes:
- Centralised dashboards: Control, incident, partnership, and improvement logs by grant or project. Live connection between asset inventory, risk updates, and actionable controls.
- Signed board reviews: Digital or paper minutes attached to every significant policy and risk update.
- Traceability tables: The ability to instantly connect an event (e.g., supplier breach) to the updated risk register, SoA control reference, and proof of remediation.
Table examples:
| Expectation | Operationalisation | ISO 27001 / Annex A Ref |
|---|---|---|
| Board risk review | Minutes, dashboard logs | Cl. 6, 9.3, Annex A.5.7 |
| Incident response | Playbook, notification | A.5.24–A.5.28 |
| Supplier due diligence | Contract & review evidence | A.5.19–A.5.22 |
| Staff awareness | Training logs | A.6.3, A.8.7 |
| Trigger/Event | Risk Change | Control / SoA Link | Evidence Logged |
|---|---|---|---|
| Phishing drill | Risk update | A.5.25/A.5.26 | Staff logs, comms |
| New vendor onboard | Review log | A.5.21/A.5.19 | Signed contract review |
Automated reporting, improvement logs, and version histories are critical-auditors expect dynamic, not static, evidence.
How can research organisations use NIS 2 compliance to gain a strategic edge, not just bear a burden?
Active, transparent compliance is fast becoming a trust accelerator-with tangible advantages for funding, partnerships, and reputation:
- Faster funding & partnerships: Evidence packs, audit dashboards, and management logs ease pre-award diligence, accelerating grant and tender wins.
- Reputational boost: Public, regulator, and peer perceptions shift toward leadership-for organisations who “live” their compliance loops.
- Resilience dividends: Staff engagement, recurring training, and visible improvement actions eliminate “surprise” findings and foster a culture of security.
- Board & leadership capital: Demonstrating engagement, approving continuous improvements, and publishing transparent logs now serve as differentiators-earning trust from regulators and peers.
Don’t treat NIS 2 as a one-off hurdle-turn it into a living framework for resilience and influence. Make every compliance record, review, and improvement part of your reputation and funding toolbox.